Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates
- PMID: 15951837
- PMCID: PMC1143588
- DOI: 10.1172/JCI23743
Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates
Abstract
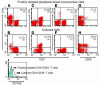
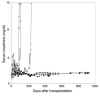
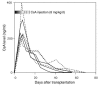
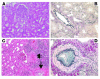
Anergic T cells generated ex vivo are reported to have immunosuppressive effects in vitro and in vivo. Here, we tested this concept in nonhuman primates. Alloreactive T cells were rendered anergic ex vivo by coculture with donor alloantigen in the presence of anti-CD80/CD86 mAbs before adoptive transfer via renal allograft to rhesus monkey recipients. The recipients were briefly treated with cyclophosphamide and cyclosporine A during the preparation of the anergic cells. Thirteen days after renal transplantation, the anergic T cells were transferred to the recipient, after which no further immunosuppressive agents were administered. Rejection-free survival was prolonged in all treated recipients, and 3 of 6 animals survived long term (410-880 days at study's end). In the long-surviving recipients, proliferative responses against alloantigen were inhibited in a donor-specific manner, and donor-type, but not third-party, skin allografts were also accepted, which demonstrated that antigen-specific tolerance had been induced. We conclude that anergic T cells generated ex vivo by blocking CD28/B7 costimulation can suppress renal allograft rejection after adoptive transfer in nonhuman primates. This strategy may be applicable to the design of safe clinical trials in humans.
Figures


Similar articles
-
Anergic T cells generated in vitro suppress rejection response to islet allografts.Transplantation. 2000 May 27;69(10):2144-8. doi: 10.1097/00007890-200005270-00032. Transplantation. 2000. PMID: 10852614
-
A combination of anergic cells' adoptive transfer and rapamycin therapy prolongs cardiac allograft survival in mice.Scand J Immunol. 2005 Mar;61(3):266-73. doi: 10.1111/j.1365-3083.2005.01555.x. Scand J Immunol. 2005. PMID: 15787744
-
Prevention of renal allograft rejection in primates by blocking the B7/CD28 pathway.Transplantation. 1999 Oct 15;68(7):1010-8. doi: 10.1097/00007890-199910150-00019. Transplantation. 1999. PMID: 10532543
-
Prolongation of renal allograft survival by anergic cells: advantages and limitations.Clin Transplant. 2010 Jul;24 Suppl 22:6-10. doi: 10.1111/j.1399-0012.2010.01269.x. Clin Transplant. 2010. PMID: 20590686 Review.
-
The mosaic of immunosuppressive drugs.Mol Immunol. 2003 Jul;39(17-18):1073-7. doi: 10.1016/s0161-5890(03)00075-0. Mol Immunol. 2003. PMID: 12835079 Review.
Cited by
-
Anti-Inflammatory Effects of Ex Vivo-Generated Donor Antigen-Specific Immunomodulatory Cells on Pancreatic Islet Transplantation.Cell Transplant. 2025 Jan-Dec;34:9636897251317887. doi: 10.1177/09636897251317887. Cell Transplant. 2025. PMID: 39981681 Free PMC article.
-
The Role of Regulatory Myeloid Cell Therapy in Renal Allograft Rejection.Front Immunol. 2021 Feb 24;12:625998. doi: 10.3389/fimmu.2021.625998. eCollection 2021. Front Immunol. 2021. PMID: 33717141 Free PMC article. Review.
-
Sequential monitoring and stability of ex vivo-expanded autologous and nonautologous regulatory T cells following infusion in nonhuman primates.Am J Transplant. 2015 May;15(5):1253-66. doi: 10.1111/ajt.13113. Epub 2015 Mar 17. Am J Transplant. 2015. PMID: 25783759 Free PMC article.
-
Expansion of CD4+CD25+ suppressive regulatory T cells from rhesus macaque peripheral blood by FN18/antihuman CD28-coated Dynal beads.Hum Immunol. 2007 Jun;68(6):478-90. doi: 10.1016/j.humimm.2007.02.011. Epub 2007 Apr 2. Hum Immunol. 2007. PMID: 17509447 Free PMC article.
-
Cell-based immunosuppression in kidney transplantation: the value of non-human primate studies.Kidney Int. 2015 Nov;88(5):1196-7. doi: 10.1038/ki.2015.262. Kidney Int. 2015. PMID: 26579685 Free PMC article. No abstract available.
References
-
- Lenschow DJ, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–791. - PubMed
-
- Lenschow DJ, et al. Inhibition of transplant rejection following treatment with anti-B7-2 and anti-B7-1 antibodies. Transplantation. 1995;60:1171–1178. - PubMed
-
- Bashuda H, et al. Specific acceptance of cardiac allograft after treatment with antibodies to CD80 and CD86 in mice. Transplant. Proc. 1996;28:1039–1041. - PubMed
-
- Larsen CP, et al. CD40-gp39 interactions play a critical role during allograft rejection. Transplantation. 1996;61:4–9. - PubMed
-
- Larsen CP, et al. Long-term acceptance of skin cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. - PubMed

