FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes
- PMID: 15951838
- PMCID: PMC1143587
- DOI: 10.1172/JCI23418
FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes
Abstract
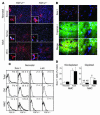
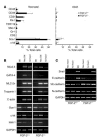
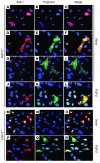
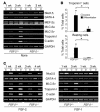
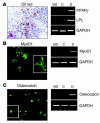
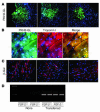
Recent evidence suggests that the heart possesses a greater regeneration capacity than previously thought. In the present study, we isolated undifferentiated precursors from the cardiac nonmyocyte cell population of neonatal hearts, expanded them in culture, and induced them to differentiate into functional cardiomyocytes. These cardiac precursors appear to express stem cell antigen-1 and demonstrate characteristics of multipotent precursors of mesodermal origin. Following infusion into normal recipients, these cells home to the heart and participate in physiological and pathophysiological cardiac remodeling. Cardiogenic differentiation in vitro and in vivo depends on FGF-2. Interestingly, this factor does not control the number of precursors but regulates the differentiation process. These findings suggest that, besides its angiogenic actions, FGF-2 could be used in vivo to facilitate the mobilization and differentiation of resident cardiac precursors in the treatment of cardiac diseases.
Figures


Similar articles
-
Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration.J Cell Sci. 2007 May 15;120(Pt 10):1791-800. doi: 10.1242/jcs.006122. J Cell Sci. 2007. PMID: 17502484
-
Isolation and expansion of resident cardiac progenitor cells.Expert Rev Cardiovasc Ther. 2007 Jan;5(1):33-43. doi: 10.1586/14779072.5.1.33. Expert Rev Cardiovasc Ther. 2007. PMID: 17187455 Review.
-
Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells.Circ Res. 2007 Jun 8;100(11):1615-25. doi: 10.1161/01.RES.0000269182.22798.d9. Epub 2007 May 3. Circ Res. 2007. PMID: 17478732
-
Sca-1 expression is associated with decreased cardiomyogenic differentiation potential of skeletal muscle-derived adult primitive cells.J Mol Cell Cardiol. 2006 Oct;41(4):650-60. doi: 10.1016/j.yjmcc.2006.07.011. Epub 2006 Aug 30. J Mol Cell Cardiol. 2006. PMID: 16938308
-
[Electrophysiological properties of stem cells].Herz. 2006 Apr;31(2):123-6. doi: 10.1007/s00059-006-2793-y. Herz. 2006. PMID: 16738835 Review. German.
Cited by
-
Stem cell therapy for chronic heart failure: an updated appraisal.Expert Opin Biol Ther. 2013 Apr;13(4):503-16. doi: 10.1517/14712598.2013.749852. Epub 2013 Jan 6. Expert Opin Biol Ther. 2013. PMID: 23289619 Free PMC article. Review.
-
Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction.J Immunol. 2015 Jan 15;194(2):499-503. doi: 10.4049/jimmunol.1401948. Epub 2014 Dec 10. J Immunol. 2015. PMID: 25505286 Free PMC article.
-
Cardiac actions of fibroblast growth factor 23.Bone. 2017 Jul;100:69-79. doi: 10.1016/j.bone.2016.10.001. Epub 2016 Oct 7. Bone. 2017. PMID: 27725315 Free PMC article. Review.
-
Endogenous retinoic acid regulates cardiac progenitor differentiation.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9234-9. doi: 10.1073/pnas.0910430107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439714 Free PMC article.
-
Controlled multiple growth factor delivery from bone tissue engineering scaffolds via designed affinity.Tissue Eng Part A. 2014 Aug;20(15-16):2077-87. doi: 10.1089/ten.tea.2013.0358. Epub 2013 Dec 18. Tissue Eng Part A. 2014. PMID: 24350567 Free PMC article.
References
-
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat. Med. 1998;4:1241–1243. - PubMed
-
- Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. - PubMed
-
- Sussman MA, Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu. Rev. Physiol. 2004;66:29–48. - PubMed
-
- Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. - PubMed
-
- Strauer BE, Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107:929–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

