Influence of supporting cells on neuronal degeneration after hair cell loss
- PMID: 15952050
- PMCID: PMC2538335
- DOI: 10.1007/s10162-004-5050-1
Influence of supporting cells on neuronal degeneration after hair cell loss
Abstract
In sensorineural hearing loss, hair cell loss is often followed by loss of cochlear nerve fibers, which can continue for years after the insult. The degree and time course of neuronal loss varies, but the reasons for this variation are unclear. The present study addresses this issue with a quantitative analysis of hair cell, supporting cell, and neuronal survival in animals with long-term survival of up to 5.5 years from two types of drug-induced hair cell loss: aminoglycoside antibiotics and platinum-containing chemotherapeutics. To complement the analysis of the effects of organ of Corti damage on neuronal survival, cases of primary neuronal degeneration, via auditory nerve section, are also assessed. Analysis shows that (1) long-term neuronal survival is enhanced when supporting cells in the inner hair cell (IHC) area remain intact; (2) after hair cell loss, the time course of neuronal loss is slower in the apex than in the base; (3) primary loss of cochlear nerve fibers does not lead to secondary degeneration of sensory cells or supporting cells in the organ of Corti; and (4) after auditory nerve section, there can be a massive reinnervation of the IHC region, especially in the apex. Results are consistent with the idea that supporting cells participate in the regulation of neuronal survival and neuronal sprouting in the organ of Corti.
Figures


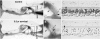

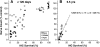

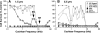
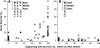


References
-
- Anniko M, Thornell LE, Gustavsson H, Virtanen I. Intermediate filaments in the newborn inner ear of the mouse. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 1986;48:98–106. - PubMed
-
- Backus RM, DeGroot JC, Tange RA, Huizing EH. Pathological findings in the human auditory system following long-standing gentamicin ototoxicity. Arch. Oto. Rhino. Laryngol. 1987;244:69–73. - PubMed
-
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary vs. secondary events. Am. J. Otol. 2000;21:505–509. - PubMed
-
- Brown MC, Berglund AM, Kiang NYS, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J. Comp. Neurol. 1988;278:581–590. - PubMed
-
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of erbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat. Neurosci. 2003;6:1186–1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

