Predicting the effect of post-implant cochlear fibrosis on residual hearing
- PMID: 15953528
- PMCID: PMC3623675
- DOI: 10.1016/j.heares.2005.03.018
Predicting the effect of post-implant cochlear fibrosis on residual hearing
Abstract
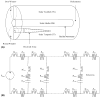
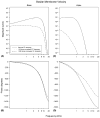
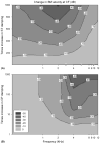
Intracochlear scarring is a well-described sequela of cochlear implantation. We developed a mathematical model of passive cochlear mechanics to predict the impact that this might have upon residual acoustical hearing after implantation. The cochlea was modeled using lumped impedance terms for scala vestibuli (SV), scala tympani (ST), and the cochlear partition (CP). The damping of ST and CP was increased in the basal one half of the cochlea to simulate the effect of scar tissue. We found that increasing the damping of the ST predominantly reduced basilar membrane vibrations in the apex of the cochlea while increasing the damping of the CP predominantly reduced basilar membrane vibrations in the base of the cochlea. As long as intracochlear scarring continues to occur with cochlear implantation, there will be limitations on hearing preservation. Newer surgical techniques and electrode technologies that do not result in as much scar tissue formation will permit improved hearing preservation.
Figures


References
-
- Alexiades G, Roland JT, Jr, Fishman AJ, Shapiro W, Waltzmann SB, Cohen NL. Cochlear reimplantation: surgical techniques and functional results. Laryngoscope. 2001;111:1608–1613. - PubMed
-
- Allen JB. Cochlear micromechanics – a physical model of transduction. J Acoust Soc Am. 1980;68:1660–1670. - PubMed
-
- Araki S, Kawano A, Seldon HL, Shepherd RK, Funasaka S, Clark GM. Effect of intracochlear factors on spiral ganglion cells and auditory brain stem response after long-term electrical stimulation in deafened kittens. Otolaryngol Head Neck Surg. 2000;122:425–433. - PubMed
-
- Boggess WJ, Baker JE, Balkany TJ. Loss of residual hearing after cochlear implantation. Laryngoscope. 1989;99:1002–1005. - PubMed
-
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

