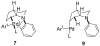Selective synthesis of 5- or 6-aryl octahydrocyclopenta[b]pyrroles from a common precursor through control of competing pathways in a Pd-catalyzed reaction
- PMID: 15954769
- PMCID: PMC2736107
- DOI: 10.1021/ja0430346
Selective synthesis of 5- or 6-aryl octahydrocyclopenta[b]pyrroles from a common precursor through control of competing pathways in a Pd-catalyzed reaction
Abstract
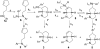
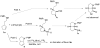
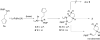
The Pd/phosphine-catalyzed reaction of 1 with aryl bromides leads to the selective synthesis of either 6-aryl octahydrocyclopenta[b]pyrroles (3), the corresponding 5-aryl isomers 5, diarylamine 2, or hexahydrocyclopenta[b]pyrrole 4 depending on the structure of the phosphine ligand. These transformations are effective with a variety of different aryl bromides and provide 3-5 with excellent levels of diastereoselectivity (dr > or = 20:1). The changes in product distribution are believed to derive from the influence of Pd-catalyst structure on the relative rates of reductive elimination, beta-hydride elimination, alkene insertion, and alkene displacement processes in a mechanistically complex reaction. The effect of phosphine ligand structure on product distribution is described in detail, along with analysis of a proposed mechanism for these transformations.
Figures
References
-
-
For recent examples, see:Kirchhoff JH, Netherton MR, Hills ID, Fu GC. J. Am. Chem. Soc. 2002;124:13662–13663.Torraca KE, Huang X, Parrish CA, Buchwald SL. J. Am. Chem. Soc. 2001;123:10770–10771.Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 1999;121:1473–1478.Lee C-W, Oh KS, Kim KS, Ahn KH. Org. Lett. 2000;2:1213–1216.Aggarwal VK, Davies PW, Moss WO. J. Chem. Soc., Chem. Commun. 2002:972–973.Coperet C, Negishi E. -i. Org. Lett. 1999;1:165–168.
-
-
-
A portion of this work has been previously communicated. See:Ney JE, Wolfe JP. Angew. Chem. Int. Ed. 2004;43:3605–3608.
-
-
-
For related transformations that provide indolines or tetrahydrofurans see:Lira R, Wolfe JP. J. Am. Chem. Soc. 2004;126:13906–13907.Wolfe JP, Rossi MA. J. Am. Chem. Soc. 2004;126:1620–1621.Hay MB, Hardin AR, Wolfe JP. J. Org. Chem. 2005;70:3099–3107.
-
-
-
For recent, complementary methods involving late transition metal-catalyzed synthesis of pyrrolidines from γ-aminoalkenes, see:Bender CF, Widenhoefer RA. J. Am. Chem. Soc. 2005;127:1070–1071.Manzoni MR, Zabawa TP, Kasi D, Chemler SR. Organometallics. 2004;23:5618–5621.
-
-
- Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131–209.
- Hartwig JF. In: Modern Arene Chemistry. Astruc D, editor. Wiley-VCH; Weinheim: 2002. p. 107.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources