Ras plasma membrane signalling platforms
- PMID: 15954863
- PMCID: PMC1184533
- DOI: 10.1042/BJ20050231
Ras plasma membrane signalling platforms
Abstract
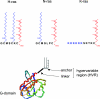
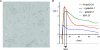
The plasma membrane is a complex, dynamic structure that provides platforms for the assembly of many signal transduction pathways. These platforms have the capacity to impose an additional level of regulation on cell signalling networks. In this review, we will consider specifically how Ras proteins interact with the plasma membrane. The focus will be on recent studies that provide novel spatial and dynamic insights into the micro-environments that different Ras proteins utilize for signal transduction. We will correlate these recent studies suggesting Ras proteins might operate within a heterogeneous plasma membrane with earlier biochemical work on Ras signal transduction.
Figures
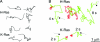

References
-
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 2003;32:257–283. - PubMed
-
- Kusumi A., Koyama-Honda I., Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. - PubMed
-
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. - PubMed
-
- Ehrhardt A., Ehrhardt G. R., Guo X., Schrader J. W. Ras and relatives – job sharing and networking keep an old family together. Exp. Hematol. 2002;30:1089–1106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

