Weaning induces NOS-2 expression through NF-kappaB modulation in the lactating mammary gland: importance of GSH
- PMID: 15954866
- PMCID: PMC1276959
- DOI: 10.1042/BJ20050507
Weaning induces NOS-2 expression through NF-kappaB modulation in the lactating mammary gland: importance of GSH
Abstract
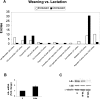
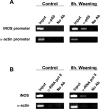
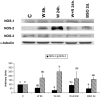
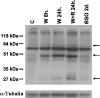
At the end of lactation the mammary gland undergoes involution, a process characterized by apoptosis of secretory cells and tissue remodelling. To gain insight into this process, we analysed the gene expression profile by oligonucleotide microarrays during lactation and after forced weaning. Up-regulation of inflammatory mediators and acute-phase response genes during weaning was found. Expression of IkappaBalpha (inhibitory kappaBalpha), a protein known to modulate NF-kappaB (nuclear factor-kappaB) nuclear translocation, was significantly up-regulated. On the other hand, there was a time-dependent degradation of IkappaBalpha protein levels in response to weaning, suggesting a role for NF-kappaB. Furthermore, we have demonstrated, using chromatin immunoprecipitation assays, binding of NF-kappaB to the NOS-2 (inducible nitric oxide synthase) promoter at the early onset of events triggered during weaning. The three isoforms of NOS are constitutively present in the lactating mammary gland; however, while NOS-2 mRNA and protein levels and, consequently, NO production are increased during weaning, NOS-3 protein levels are diminished. Western blot analyses have demonstrated that protein nitration is increased in the mammary gland during weaning, but this is limited to a few specific tyrosine-nitrated proteins. Interestingly, inhibition of GSH synthesis at the peak of lactation partially mimics these findings, highlighting the role of NO production and GSH depletion during involution.
Figures
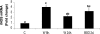
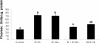
References
-
- Visvader J. E., Lindeman G. J. Transcriptional regulators in mammary gland development and cancer. Int. J. Biochem. Cell Biol. 2003;37:1034–1051. - PubMed
-
- Clarkson R. W. E., Watson C. J. Microarray analysis of the involution switch. J. Mammary Gland Biol. Neoplasia. 2003;8:309–319. - PubMed
-
- Stein T., Morris J. S., Davies C. R., Weber-Hall S. J., Duffy M.-A., Heath V. J., Bell A. K., Ferrier R. K., Sandilands G. P., Gusterson B. A. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–R91. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

