Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors
- PMID: 15955837
- PMCID: PMC2212026
- DOI: 10.1084/jem.20042407
Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors
Abstract
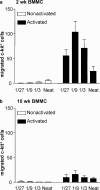
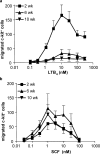
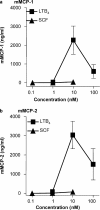
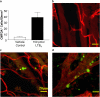
Mast cells are tissue-resident cells with important functions in allergy and inflammation. Pluripotential hematopoietic stem cells in the bone marrow give rise to committed mast cell progenitors that transit via the blood to tissues throughout the body, where they mature. Knowledge is limited about the factors that release mast cell progenitors from the bone marrow or recruit them to remote tissues. Mouse femoral bone marrow cells were cultured with IL-3 for 2 wk and a range of chemotactic agents were tested on the c-kit(+) population. Cells were remarkably refractory and no chemotaxis was induced by any chemokines tested. However, supernatants from activated mature mast cells induced pronounced chemotaxis, with the active principle identified as leukotriene (LT) B(4). Other activation products were inactive. LTB(4) was highly chemotactic for 2-wk-old cells, but not mature cells, correlating with a loss of mRNA for the LTB(4) receptor, BLT1. Immature cells also accumulated in vivo in response to intradermally injected LTB(4). Furthermore, LTB(4) was highly potent in attracting mast cell progenitors from freshly isolated bone marrow cell suspensions. Finally, LTB(4) was a potent chemoattractant for human cord blood-derived immature, but not mature, mast cells. These results suggest an autocrine role for LTB(4) in regulating tissue mast cell numbers.
Figures




Similar articles
-
Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells.J Immunol. 2006 Sep 1;177(5):3439-47. doi: 10.4049/jimmunol.177.5.3439. J Immunol. 2006. PMID: 16920986
-
Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3.Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11712-7. doi: 10.1073/pnas.0701700104. Epub 2007 Jul 2. Proc Natl Acad Sci U S A. 2007. PMID: 17606905 Free PMC article.
-
Cytochrome P-450 4F18 is the leukotriene B4 omega-1/omega-2 hydroxylase in mouse polymorphonuclear leukocytes: identification as the functional orthologue of human polymorphonuclear leukocyte CYP4F3A in the down-regulation of responses to LTB4.J Biol Chem. 2006 Mar 17;281(11):7189-96. doi: 10.1074/jbc.M513101200. Epub 2005 Dec 27. J Biol Chem. 2006. PMID: 16380383
-
The role of leukotriene B(4) in allergic diseases.Allergol Int. 2008 Dec;57(4):291-8. doi: 10.2332/allergolint.08-RAI-0019. Epub 2008 Dec 1. Allergol Int. 2008. PMID: 18797182 Review.
-
Origin, maturation and recruitment of mast cell precursors.Front Biosci (Schol Ed). 2011 Jun 1;3(4):1390-406. doi: 10.2741/231. Front Biosci (Schol Ed). 2011. PMID: 21622276 Review.
Cited by
-
Biomarkers of Autoimmune Chronic Spontaneous Urticaria.Curr Allergy Asthma Rep. 2023 Dec;23(12):655-664. doi: 10.1007/s11882-023-01117-7. Epub 2023 Dec 8. Curr Allergy Asthma Rep. 2023. PMID: 38064133 Review.
-
Distinguishing Mast Cell Progenitors from Mature Mast Cells in Mice.Stem Cells Dev. 2015 Jul 15;24(14):1703-11. doi: 10.1089/scd.2014.0553. Epub 2015 Apr 13. Stem Cells Dev. 2015. PMID: 25744159 Free PMC article.
-
Genetic evidence for critical roles of P38α protein in regulating mast cell differentiation and chemotaxis through distinct mechanisms.J Biol Chem. 2012 Jun 8;287(24):20258-69. doi: 10.1074/jbc.M112.358119. Epub 2012 Apr 19. J Biol Chem. 2012. PMID: 22518842 Free PMC article.
-
Constitutive androstane receptor agonist, TCPOBOP, attenuates steatohepatitis in the methionine choline-deficient diet-fed mouse.World J Gastroenterol. 2007 Nov 14;13(42):5635-41. doi: 10.3748/wjg.v13.i42.5635. World J Gastroenterol. 2007. PMID: 17948939 Free PMC article.
-
Mast cell repopulation of the peritoneal cavity: contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors.BMC Immunol. 2010 Jun 24;11:32. doi: 10.1186/1471-2172-11-32. BMC Immunol. 2010. PMID: 20576124 Free PMC article.
References
-
- Marshall, J.S. 2004. Mast-cell responses to pathogens. Nat. Rev. Immunol. 4:787–799. - PubMed
-
- Kitamura, Y., S. Go, and K. Hatanaka. 1978. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 52:447–452. - PubMed
-
- Wong, G.W., S. Yasuda, N. Morokawa, L. Li, and R.L. Stevens. 2004. Mouse chromosome 17A3.3 contains 13 genes that encode functional tryptic-like serine proteases with distinct tissue and cell expression patterns. J. Biol. Chem. 279:2438–2452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

