Maturation of the ground tissue of the root is regulated by gibberellin and SCARECROW and requires SHORT-ROOT
- PMID: 15955927
- PMCID: PMC1150384
- DOI: 10.1104/pp.104.058362
Maturation of the ground tissue of the root is regulated by gibberellin and SCARECROW and requires SHORT-ROOT
Figures
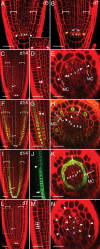

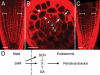
References
-
- Barley R, Waites R (2002) Plant meristems: the interplay of KNOX and gibberellins. Curr Biol 12: R696–R698 - PubMed
-
- Baum SF, Dubrovsky JG, Rost TL (2002) Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am J Bot 89: 908–920 - PubMed
-
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser M-T, Aeschbacher RA (1993) Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development 119: 57–70 - PubMed
-
- Benfey PN, Scheres B (2000) Root development. Curr Biol 10: R813–R815 - PubMed
-
- Doerner P (2003) Plant meristems: a merry-go-round of signals. Curr Biol 13: R368–R374 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

