Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention
- PMID: 15956187
- PMCID: PMC1166599
- DOI: 10.1073/pnas.0501744102
Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention
Abstract
The peroxisome proliferator-activated receptor subtype gamma (PPARgamma) ligands, namely the synthetic insulin-sensitizing thiazolidinedione (TZD) compounds, have demonstrated great potential in the treatment of type II diabetes. However, their clinical applicability is limited by a common and serious side effect of edema. To address the mechanism of TZD-induced edema, we generated mice with collecting duct (CD)-specific disruption of the PPARgamma gene. We found that mice with CD knockout of this receptor were resistant to the rosiglitazone- (RGZ) induced increases in body weight and plasma volume expansion found in control mice expressing PPARgamma in the CD. RGZ reduced urinary sodium excretion in control and not in conditional knockout mice. Furthermore, RGZ stimulated sodium transport in primary cultures of CD cells expressing PPARgamma and not in cells lacking this receptor. These findings demonstrate a PPARgamma-dependent pathway in regulation of sodium transport in the CD that underlies TZD-induced fluid retention.
Figures
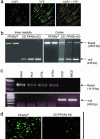
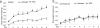
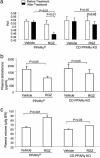
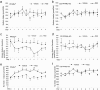
References
-
- Cheng-Lai, A. & Levine, A. (2000) Heart Dis. 2, 326-333. - PubMed
-
- Parulkar, A. A., Pendergrass, M. L., Granda-Ayala, R., Lee, T. R. & Fonseca, V. A. (2001) Ann. Intern. Med. 134, 61-71. - PubMed
-
- Haffner, S. M., Greenberg, A. S., Weston, W. M., Chen, H., Williams, K. & Freed, M. I. (2002) Circulation 106, 679-684. - PubMed
-
- Hirsch, I. B., Kelly, J. & Cooper, S. (1999) Arch. Intern. Med. 159, 1811. - PubMed
-
- Fuchtenbusch, M., Standl, E. & Schatz, H. (2000) Exp. Clin. Endocrinol. Diabetes 108, 151-163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

