Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production
- PMID: 15956283
- PMCID: PMC1895265
- DOI: 10.1182/blood-2005-03-1239
Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production
Abstract
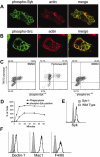
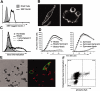
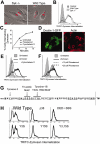
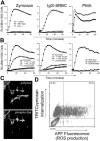
Dectin-1 is a lectin receptor for beta-glucan that is important for innate macrophage recognition of fungi and contributes to phagocytosis, reactive oxygen production, and induction of inflammatory cytokines. The mechanisms by which Dectin-1 mediates intracellular signaling are just beginning to be defined. Spleen tyrosine kinase (Syk) is a protein tyrosine kinase that is critical for adaptive immune responses where it mediates signaling through B-cell receptors, T-cell receptors, and Fc receptors. Here we report that Dectin-1 activates Syk in macrophages and is important for Dectin-1-stimulated reactive oxygen production, but not for phagocytosis. Syk activation is restricted to a subpopulation of macrophages that is in equilibrium with cells that cannot activate the pathway. The proportion of macrophages using this signaling pathway can be modulated by cytokine treatment. Thus, Dectin-1 signaling reveals dynamic macrophage heterogeneity in inflammatory activation potential.
Figures

References
-
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20: 197-216. - PubMed
-
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17: 1-14. - PubMed
-
- Chu DH, Morita CT, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165: 167-180. - PubMed
-
- Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VL. Tyrosine kinase SYK: essential functions for immunoreceptor signaling. Immunol Today. 2000;21: 148-154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

