Use of a BJAB-derived cell line for isolation of human herpesvirus 8
- PMID: 15956410
- PMCID: PMC1151914
- DOI: 10.1128/JCM.43.6.2866-2875.2005
Use of a BJAB-derived cell line for isolation of human herpesvirus 8
Abstract
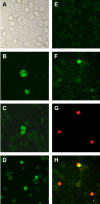
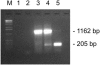
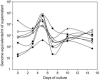
Establishment of latently infected cell lines from primary effusion lymphomas (PEL) presently is the most efficient system for the propagation of clinical strains of human herpesvirus 8 (HHV-8) in culture. Here we describe a new approach to culture productively replicating HHV-8 from patient samples. A BJAB-derived B-cell line, BBF, was found to retain HHV-8 longer, to support the latent and lytic replication programs, and to produce transmissible virus. Supernatants from n-butyrate-treated peripheral blood mononuclear cells of 24 HHV-8-seropositive renal transplant recipients were used to infect BBF cells, and replicating virus was detected in cultures from 11 patients. Moreover, BBF cells infected with saliva strains showed a highly productive profile regardless of the initial viral load, which confirms that infectious HHV-8 can be present in saliva and also suggests that saliva strains may exhibit a high tropism for B lymphocytes. In conclusion, we established an in vitro system that efficiently detects HHV-8 in samples with low viral loads and that produces infectious progeny. BBF cells can be used to propagate HHV-8 from different biological samples as well as to clarify important issues related to virus-cell interactions in a context distinct from endothelial and PEL-derived cell lines.
Figures


Similar articles
-
Evidence for both lytic replication and tightly regulated human herpesvirus 8 latency in circulating mononuclear cells, with virus loads frequently below common thresholds of detection.J Virol. 2004 Nov;78(21):11707-14. doi: 10.1128/JVI.78.21.11707-11714.2004. J Virol. 2004. PMID: 15479812 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 infection in reactive lymphoid tissues: a model for KSHV/HHV-8-related lymphomas?Hum Pathol. 2006 Jan;37(1):23-31. doi: 10.1016/j.humpath.2005.08.020. Hum Pathol. 2006. PMID: 16360412
-
Persistent infection of Epstein-Barr virus-positive B lymphocytes by human herpesvirus 8.J Virol. 1998 Oct;72(10):8143-9. doi: 10.1128/JVI.72.10.8143-8149.1998. J Virol. 1998. PMID: 9733855 Free PMC article.
-
Human herpesvirus 8 virology, epidemiology and related diseases.Jpn J Infect Dis. 2000 Aug;53(4):137-55. Jpn J Infect Dis. 2000. PMID: 11056556 Review.
-
[The discovery of three novel human viruses, human herpesviruses 6, 7, and 8].Bull Acad Natl Med. 1997 Jun-Jul;181(6):1009-22. Bull Acad Natl Med. 1997. PMID: 9453828 Review. French.
Cited by
-
Human herpesvirus-8 infection leads to expansion of the preimmune/natural effector B cell compartment.PLoS One. 2010 Nov 29;5(11):e15029. doi: 10.1371/journal.pone.0015029. PLoS One. 2010. PMID: 21124778 Free PMC article.
-
Circulating miRNA-375 as a potential novel biomarker for active Kaposi's sarcoma in AIDS patients.J Cell Mol Med. 2019 Feb;23(2):1486-1494. doi: 10.1111/jcmm.14054. Epub 2018 Dec 13. J Cell Mol Med. 2019. PMID: 30549196 Free PMC article.
-
In vitro and in vivo human herpesvirus 8 infection of placenta.PLoS One. 2008;3(12):e4073. doi: 10.1371/journal.pone.0004073. Epub 2008 Dec 30. PLoS One. 2008. PMID: 19115001 Free PMC article.
-
Crosstalk between the mesothelium and lymphomatous cells: insight into the mechanisms involved in the progression of body cavity lymphomas.Cancer Med. 2014 Feb;3(1):1-13. doi: 10.1002/cam4.159. Epub 2013 Nov 19. Cancer Med. 2014. PMID: 24402744 Free PMC article.
-
Predictors of immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma: a case report.Infect Agent Cancer. 2016 Feb 3;11:5. doi: 10.1186/s13027-016-0051-3. eCollection 2016. Infect Agent Cancer. 2016. PMID: 26848307 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Bigoni, B., R. Dolcetti, L. de Lellis, A. Carbone, M. Boiocchi, E. Cassai, and D. Di Luca. 1996. Human herpesvirus 8 is present in the lymphoid system of healthy persons and can reactivate in the course of AIDS. J. Infect. Dis. 173:542-549. - PubMed
-
- Blackbourn, D. J., E. Lennette, B. Klencke, A. Moses, B. Chandran, M. Weinstein, R. G. Glogau, M. H. Witte, D. L. Way, T. Kutzkey, B. Herndier, and J. A. Levy. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 14:1123-1133. - PubMed
-
- Blackbourn, D. J., J. Ambroziak, E. Lennette, M. Adams, B. Ramachandran, and J. A. Levy. 1997. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 349:609-611. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

