Differential sensitivity of murine leukemia virus to APOBEC3-mediated inhibition is governed by virion exclusion
- PMID: 15956565
- PMCID: PMC1143768
- DOI: 10.1128/JVI.79.13.8201-8207.2005
Differential sensitivity of murine leukemia virus to APOBEC3-mediated inhibition is governed by virion exclusion
Abstract
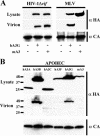
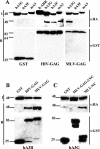
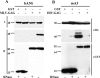
While members of the APOBEC3 family of human intrinsic resistance factors are able to restrict the replication of Vif-deficient forms of human immunodeficiency virus type 1 (HIV-1), they are unable to block replication of wild-type HIV-1 due to the action of Vif, which induces their degradation. In contrast, HIV-1 Vif is unable to block inhibition mediated by APOBEC3 proteins expressed by several heterologous species, including mice. Here, we have asked whether the simple retrovirus murine leukemia virus (MLV) is sensitive to restriction by the cognate murine or heterologous, human APOBEC3 proteins. We demonstrate that MLV is highly sensitive to inhibition by human APOBEC3G and APOBEC3B but resistant to inhibition by murine APOBEC3 or by other human APOBEC3 proteins, including APOBEC3F. This sensitivity fully correlates with the ability of these proteins to be packaged into MLV virion particles: i.e., human APOBEC3G and APOBEC3B are packaged while murine APOBEC3 and human APOBEC3F are excluded. Moreover, this packaging in turn correlates with the differential ability of these APOBEC3 proteins to bind MLV Gag. Together, these data suggest that MLV Gag has evolved to avoid binding, and hence virion packaging, of the cognate murine APOBEC3 protein but that MLV infectivity is still restricted by certain heterologous APOBEC3 proteins that retain this ability. Moreover, these results suggest that APOBEC3 proteins may help prevent the zoonotic infection of humans by simple retroviruses and provide a mechanism for how simple retroviruses can avoid inhibition by APOBEC3 family members.
Figures



References
-
- Alce, T. M., and W. Popik. 2004. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J. Biol. Chem. 279:34083-34086. - PubMed
-
- Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 11:1109-1115. - PubMed
-
- Bishop, K. N., R. K. Holmes, A. M. Sheehy, N. O. Davidson, S.-J. Cho, and M. H. Malim. 2004. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 14:1392-1396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

