An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies
- PMID: 15956567
- PMCID: PMC1143705
- DOI: 10.1128/JVI.79.13.8217-8229.2005
An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies
Abstract
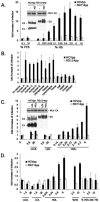
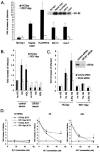
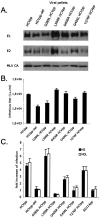
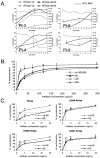
Hepatitis C virus (HCV) circulates in the bloodstream in different forms, including complexes with immunoglobulins and/or lipoproteins. To address the significance of such associations, we produced or treated HCV pseudoparticles (HCVpp), a valid model of HCV cell entry and its inhibition, with naïve or patient-derived sera. We demonstrate that infection of hepatocarcinoma cells by HCVpp is increased more than 10-fold by human serum factors, of which high-density lipoprotein (HDL) is a major component. Infection enhancement requires scavenger receptor BI, a molecule known to mediate HDL uptake into cells as well as HCVpp entry, and involves conserved amino acid positions in hypervariable region 1 (HVR1) of the E2 glycoprotein. Additionally, we show that the interaction with human serum or HDL, but not with low-density lipoprotein, leads to the protection of HCVpp from neutralizing antibodies, including monoclonal antibodies and antibodies present in patient sera. Finally, the deletion or mutation of HVR1 in HCVpp abolishes infection enhancement and leads to increased sensitivity to neutralizing antibodies/sera compared to that of parental HCVpp. Altogether, these results assign to HVR1 new roles which are complementary in helping HCV to survive within its host. Besides immune escape by mutation, HRV1 can mediate the enhancement of cell entry and the protection of virions from neutralizing antibodies. By preserving a balance between these functions, HVR1 may be essential for the viral persistence of HCV.
Figures
References
-
- Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 100:14199-14204. - PMC - PubMed
-
- Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F.-L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 278:41624-41630. - PubMed
-
- Booth, J. C., U. Kumar, D. Webster, J. Monjardino, and H. C. Thomas. 1998. Comparison of the rate of sequence variation in the hypervariable region of E2/NS1 region of hepatitis C virus in normal and hypogammaglobulinemic patients. Hepatology 27:223-227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

