Innate STAT1-dependent genomic response of neurons to the antiviral cytokine alpha interferon
- PMID: 15956575
- PMCID: PMC1143744
- DOI: 10.1128/JVI.79.13.8295-8302.2005
Innate STAT1-dependent genomic response of neurons to the antiviral cytokine alpha interferon
Abstract
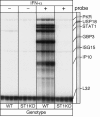
Alpha/beta interferons (IFNs-alpha/beta) are cytokines that play an essential role in the host defense against viral infection. Our previous studies have shown that the key IFN signaling molecule STAT1 is highly elevated and activated in central nervous system neurons during viral infection and in transgenic mice with astrocyte production of IFN-alpha (glial fibrillary acidic protein [GFAP]-IFN-alpha), suggesting that neurons are a very responsive target cell population for IFNs. To elucidate the genomic response of neurons to IFN-alpha, we undertook studies both in vitro and in vivo. Gene chip analysis was applied to RNA from IFN-alpha-treated or untreated primary cortical neuronal cultures derived from embryonic day 15 fetal wild-type or STAT1 knockout (KO) mice. The expression of 51 known and 5 unknown genes was increased significantly by more than twofold after exposure of wild-type but not STAT1 KO neurons to IFN-alpha. Some more highly expressed genes included IFN-induced 15-kDa protein, ubiquitin-specific protease 18, glucocorticoid attenuated response genes, IFN-induced GTPases, and the chemokine CXCL10. For several of these genes, the gene chip findings were confirmed by RNase protection assays. In addition, examination of the expression of some of these selected genes revealed that they were increased in neurons in the brain of either GFAP-IFN-alpha mice or mice infected with lymphocytic choriomeningitis virus. In conclusion, our study revealed a robust STAT1-dependent genomic response of neurons to IFN-alpha, highlighting an innate potential of these cells to defend against viral infection in the brain.
Figures


References
-
- Akwa, Y., D. E. Hassett, M. L. Eloranta, K. Sandberg, E. Masliah, H. Powell, J. L. Whitton, F. E. Bloom, and I. L. Campbell. 1998. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J. Immunol. 161:5016-5026. - PubMed
-
- Arcellana-Panlilio, M., and S. M. Robbins. 2002. Cutting-edge technology. I. Global gene expression profiling using DNA microarrays. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G397-G402. - PubMed
-
- Asensio, V. C., C. Kincaid, and I. L. Campbell. 1999. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J. Neurovirol. 5:65-75. - PubMed
-
- Blatch, G. L., and M. Lassle. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21:932-939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

