Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors
- PMID: 15956585
- PMCID: PMC1143763
- DOI: 10.1128/JVI.79.13.8410-8421.2005
Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors
Abstract
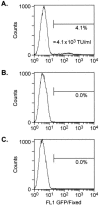
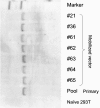
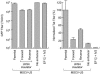
Permanent genetic modification of replicating primitive hematopoietic cells by an integrated vector has many potential therapeutic applications. Both oncoretroviral and lentiviral vectors have a predilection for integration into transcriptionally active genes, creating the potential for promoter activation or gene disruption. The use of self-inactivating (SIN) vectors in which a deletion of the enhancer and promoter sequences from the 3' long terminal repeat (LTR) is copied over into the 5' LTR during vector integration is designed to improve safety by reducing the risk of mobilization of the vector genome and the influence of the LTR on nearby cellular promoters. Our results indicate that SIN vectors are mobilized in cells expressing lentiviral proteins, with the frequency of mobilization influenced by features of the vector design. The mechanism of transcription of integrated vector genomes was evaluated using a promoter trap design with a vector encoding tat but lacking an upstream promoter in a cell line in which drug resistance depended on tat expression. In six clones studied, all transcripts originated from cryptic promoters either upstream or within the vector genome. We estimate that approximately 1 in 3,000 integrated vector genomes is transcribed, leading to the inference that activation of cryptic promoters must depend on local features of chromatin structure and the constellation of nearby regulatory elements as well as the nature of the regulatory elements within the vector.
Figures






References
-
- Ailles, L. E., and L. Naldini. 2002. HIV-1-derived lentiviral vectors. Curr. Top. Microbiol. Immunol. 261:31-52. - PubMed
-
- Allan, M., W. G. Lanyon, and J. Paul. 1983. Multiple origins of transcription in the 4.5 kb upstream of the epsilon-globin gene. Cell 35:187-197. - PubMed
-
- Chen, W. V., J. Delrow, P. D. Corrin, J. P. Frazier, and P. Soriano. 2004. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat. Genet. 36:304-312. - PubMed
-
- Evans, J. T., and J. V. Garcia. 2000. Lentivirus vector mobilization and spread by human immunodeficiency virus. Hum. Gene Ther. 11:2331-2339. - PubMed
-
- Flint, S. J., L. W. Enquist, R. M. Krug, V. R. Racaniello, and A. M. Skalka (ed.) 2000. Principles of virology: molecular biology, pathogenesis, and control. ASM Press, Washington, D.C.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

