Pharmacokinetically guided phase I trial of topotecan and etoposide phosphate in recurrent ovarian cancer
- PMID: 15956976
- PMCID: PMC2361471
- DOI: 10.1038/sj.bjc.6602657
Pharmacokinetically guided phase I trial of topotecan and etoposide phosphate in recurrent ovarian cancer
Abstract
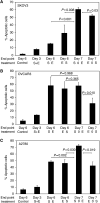
A pharmacokinetically guided phase I study of topotecan and etoposide phosphate was conducted in recurrent ovarian cancer. The scheduling of the topoisomerase I and II inhibitors was determined using in vitro activity data. All patients had recurrent disease following prior platinum-containing chemotherapy. Patients had a World Health Organisation performance status of 0-2 and adequate bone marrow, renal and hepatic function. Treatment was with topotecan intravenously for 5 days followed immediately by a 5-day intravenous infusion of etoposide phosphate (EP), with pharmacokinetically guided dose adjustment. Plasma etoposide levels were measured on days 2 and 4 of the infusion. A total of 21 patients entered the study. In all, 48% were platinum resistant and 71% had received prior paclitaxel. The main toxicities were haematological, short lived and reversible. A total of 29% of patients experienced grade 4 thrombocytopenia and 66% grade 4 neutropenia after the first cycle. Neutropenia and thrombocytopenia was dose limiting. The maximum-tolerated dose was topotecan 0.85 mg m(-2) day(-1) days 1-5 followed immediately by a 5-day infusion of EP at a plasma concentration of 1 mug ml(-1). The response rate (RR) was 28% in 18 evaluable patients. There was marked interpatient variability in topoisomerase IIalpha levels measured from peripheral lymphocytes, with no observed increase following topotecan. This regimen of topotecan followed by EP demonstrated good activity in recurrent ovarian cancer and was noncrossresistant with paclitaxel. Both the toxicity and RR was higher than would be expected from the single agent data, in keeping with synergy of action.
Figures

References
-
- Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K (1994) Current Protocols In Molecular Biology. New York: John Wiley & Sons
-
- Bertrand R, O'Connor PM, Kerrigan D, Pommier Y (1992) Sequential administration of camptothecin and etoposide circumvents the antagonistic cytotoxicity of simultaneous drug administration in slowly growing human colon carcinoma HT-29 cells. Eur J Cancer 28: 743–748 - PubMed
-
- Bonner JA, Kozelsky TF (1996) The significance of the sequence of administration of topotecan and etoposide. Cancer Chemother Pharmacol 39: 109–112 - PubMed
-
- Bookman MA, Malmstrom H, Bolis G, Gordon A, Lissoni A, Krebs JB, Fields SZ (1998) Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol 16: 3345–3352 - PubMed
-
- Braybrooke JP, Levitt NC, Joel S, Davis T, Madhusudan S, Turley H, Wilner S, Harris AL, Talbot DC (2003) Pharmacokinetic study of cisplatin and infusional etoposide phosphate in advanced breast cancer with correlation of response to topoisomerase IIalpha expression. Clin Cancer Res 9: 4682–4688 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

