TEL/ETV6 accelerates erythroid differentiation and inhibits megakaryocytic maturation in a human leukemia cell line UT-7/GM
- PMID: 15958056
- PMCID: PMC11159770
- DOI: 10.1111/j.1349-7006.2005.00052.x
TEL/ETV6 accelerates erythroid differentiation and inhibits megakaryocytic maturation in a human leukemia cell line UT-7/GM
Abstract
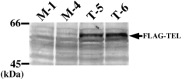
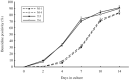
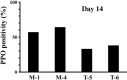
TEL/ETV6 accelerates erythroid differentiation in the murine erythroleukemia cell line. To clarify the effects of TEL on megakaryocytic maturation as well as erythroid differentiation, we chose the human leukemia cell line UT-7/GM that differentiates into the erythroid and megakaryocytic lineages by treatment with erythropoietin and thrombopoietin, respectively. Upon erythropoietin exposure, overexpressed TEL stimulated hemoglobin synthesis and accumulation of the erythroid differentiation-specific transcripts such as gamma-globin, delta-aminolevulinic acid synthase-erythroid, and erythropoietin receptor. Moreover, the glycophorin A(+)/glycoprotein IIb(-) fraction appeared more rapidly in the TEL-overexpressing cells. Interestingly, overexpression of TEL was associated with lower levels of the megakaryocytic maturation-specific glycoprotein IIb and platelet factor 4 transcripts under the treatment with thrombopoietin. Consistently, the glycophorin A(-)/glycoprotein IIb(+) fraction increased more slowly in the TEL-overexpressing cells. Finally, expression of endogenous TEL proteins in UT-7/GM cells was down-regulated following erythropoietin and thrombopoietin exposure. All these data suggest that TEL may decide the fate of human erythrocyte/megakaryocyte common progenitors to differentiate towards the erythroid lineage and against the megakaryocytic lineage.
Figures





Similar articles
-
A functional role of mitogen-activated protein kinases, erk1 and erk2, in the differentiation of a human leukemia cell line, UT-7/GM: a possible key factor for cell fate determination toward erythroid and megakaryocytic lineages.Int J Hematol. 2001 Jan;73(1):78-83. doi: 10.1007/BF02981906. Int J Hematol. 2001. PMID: 11372759
-
In vitro development of erythroid and megakaryocytic cells from a UT-7 subline, UT-7/GM.Blood. 1997 Jun 1;89(11):4021-33. Blood. 1997. PMID: 9166841
-
Establishment and characterization of the thrombopoietin-dependent megakaryocytic cell line, UT-7/TPO.Blood. 1996 Jun 1;87(11):4552-60. Blood. 1996. PMID: 8639823
-
Use of human leukemia-lymphoma cell lines in hematological research: effects of thrombopoietin on human leukemia cell lines.Hum Cell. 1996 Dec;9(4):309-16. Hum Cell. 1996. PMID: 9183663 Review.
-
Thrombopoietin: expression of its receptor MPL and proliferative effects on leukemic cells.Leukemia. 1996 Sep;10(9):1405-21. Leukemia. 1996. PMID: 8751457 Review.
Cited by
-
Leukemia-related transcription factor TEL/ETV6 expands erythroid precursors and stimulates hemoglobin synthesis.Cancer Sci. 2009 Apr;100(4):689-97. doi: 10.1111/j.1349-7006.2009.01097.x. Epub 2009 Mar 11. Cancer Sci. 2009. PMID: 19302286 Free PMC article.
-
ETV6 in hematopoiesis and leukemia predisposition.Semin Hematol. 2017 Apr;54(2):98-104. doi: 10.1053/j.seminhematol.2017.04.005. Epub 2017 Apr 7. Semin Hematol. 2017. PMID: 28637624 Free PMC article. Review.
-
Blastic plasmacytoid dendritic cell neoplasm with leukemic manifestation and ETV6 gene rearrangement: A case report.Exp Ther Med. 2015 Apr;9(4):1109-1112. doi: 10.3892/etm.2015.2236. Epub 2015 Jan 29. Exp Ther Med. 2015. PMID: 25780395 Free PMC article.
-
TEL/ETV6 binds to corepressor KAP1 via the HLH domain.Int J Hematol. 2006 Nov;84(4):377-80. doi: 10.1532/IJH97.06151. Int J Hematol. 2006. PMID: 17118767 No abstract available.
-
Significance of ETV6 rearrangement in acute promyelocytic leukemia with t(15;17)/promyelocytic leukemia/retinoic acid receptor alpha.Oncol Lett. 2016 Jun;11(6):3953-3960. doi: 10.3892/ol.2016.4544. Epub 2016 May 6. Oncol Lett. 2016. PMID: 27313723 Free PMC article.
References
-
- Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor β to a novel ets‐like gene, Tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994;. 77: 307–316. - PubMed
-
- Kwiatkowski BA, Bastian LS, Bauer TR, Jr , Tsai S, Zielinska‐Kwiatkowska AG, Hickstein DD. The ets family member Tel binds to the Fli‐1 oncoprotein and inhibits its transcriptional activity. J Biol Chem 1998; 273: 17525–30. - PubMed
-
- Potter MD, Buijs A, Kreider B, Van Rompaey L, Grosveld GC. Identification and characterization of a new human ETS‐family transcription factor, TEL2, that is expressed in hematopoietic tissues and can associate with TEL1/ETV6 . Blood 2000; 95: 3341–8. - PubMed
-
- Wang L, Hiebert SW. TEL contacts multiple co‐repressors and specifically associates with histone deacetylase‐3. Oncogene 2001; 20: 3716–25. - PubMed
-
- Martinez R, Golub TR. Blood 2000; 96: 453a.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

