Acellular Bordetella pertussis vaccine enhances mucosal interleukin-10 production, induces apoptosis of activated Th1 cells and attenuates colitis in Galphai2-deficient mice
- PMID: 15958068
- PMCID: PMC1809410
- DOI: 10.1111/j.1365-2249.2005.02807.x
Acellular Bordetella pertussis vaccine enhances mucosal interleukin-10 production, induces apoptosis of activated Th1 cells and attenuates colitis in Galphai2-deficient mice
Abstract
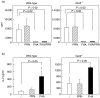
Mice deficient for the inhibitory G protein subunit alpha2 (Galphai2(-/-)) spontaneously develop a progressive inflammatory bowel disease resembling ulcerative colitis, and have a T helper 1 (Th1)-dominated immune response prior to onset of colitis, which is further augmented after the onset of disease. The present study was performed to investigate whether the Galphai2(-/-) mice were able to down-regulate the Th1-dominated inflammatory mucosal immune response and/or induce an anti-inflammatory Th2/T regulatory response and thereby diminish the severity of colitis following treatment with acellular Bordetella pertussis vaccine. The acellular vaccine against B. pertussis, the causative agent of whooping cough, has been demonstrated to induce a Th2-mediated response in both man and mice. We therefore treated Galphai2(-/-) mice intraperitoneally with a three-component acellular B. pertussis vaccine. The treated Galphai2(-/-) mice showed significantly increased interleukin (IL)-10 production in intestinal tissue, associated with significantly reduced colitis and decreased mortality, compared to untreated Galphai2(-/-) mice. The attenuation of colitis in Galphai2(-/-) mice was due, at least partly, to the B. pertussis surface antigen filamentous haemagglutinin (FHA), which almost completely inhibited proliferation of CD4(+) T cells and stimulated apoptosis of activated CD4(+) T helper 1 cells. In conclusion, the three-component acellular B. pertussis vaccine containing filamentous haemagglutinin increases the production of IL-10 in the intestinal mucosa, induces apoptosis of activated Th1 cells and attenuates colitis in Galphai2(-/-) mice.
Figures




Similar articles
-
G(alpha)i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines.J Immunol. 1997 Feb 1;158(3):1068-77. J Immunol. 1997. PMID: 9013944
-
Immunogenicity and protective efficacy of a recombinant filamentous haemagglutinin from Bordetella pertussis.Clin Exp Immunol. 2006 Jun;144(3):543-51. doi: 10.1111/j.1365-2249.2006.03097.x. Clin Exp Immunol. 2006. PMID: 16734625 Free PMC article.
-
Bordetella pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice.Dev Biol Stand. 1997;89:297-305. Dev Biol Stand. 1997. PMID: 9272363
-
New mediators of immunity and inflammation in inflammatory bowel disease.Curr Opin Gastroenterol. 2006 Jul;22(4):361-4. doi: 10.1097/01.mog.0000231808.10773.8e. Curr Opin Gastroenterol. 2006. PMID: 16760750 Review.
-
Type 1 and 2 T helper cell-mediated colitis.Curr Opin Gastroenterol. 2006 Nov;22(6):651-7. doi: 10.1097/01.mog.0000245545.80160.0f. Curr Opin Gastroenterol. 2006. PMID: 17053444 Review.
Cited by
-
Effect of live Salmonella Ty21a in dextran sulfate sodium-induced colitis.Drug Target Insights. 2007;2:221-8. doi: 10.4137/dti.s220. Epub 2007 Sep 18. Drug Target Insights. 2007. PMID: 21901076 Free PMC article.
-
Animal models of ulcerative colitis and their application in drug research.Drug Des Devel Ther. 2013 Nov 12;7:1341-57. doi: 10.2147/DDDT.S40107. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24250223 Free PMC article. Review.
References
-
- Rudolph U, Finegold MJ, Rich SS, et al. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–50. - PubMed
-
- Mariani P, Bachetoni A, D’Alessandro M, Lomanto D, Mazzocchi P, Speranza V. Effector Th-1 cells with cytotoxic function in the intestinal lamina propria of patients with Crohn's disease. Dig Dis Sci. 2000;45:2029–35. - PubMed
-
- Mullin GE, Lazenby AJ, Harris ML, Bayless TM, James SP. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not ulcerative colitis. Gastroenterology. 1992;102:1620–7. - PubMed
-
- Plevy SE, Landers CJ, Prehn J, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997;159:6276–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

