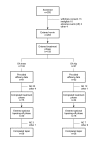
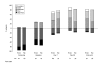Transdermal fentanyl for the treatment of pain caused by osteoarthritis of the knee or hip: an open, multicentre study
- PMID: 15958159
- PMCID: PMC1181817
- DOI: 10.1186/1471-2474-6-31
Transdermal fentanyl for the treatment of pain caused by osteoarthritis of the knee or hip: an open, multicentre study
Abstract
Background: This study was designed to evaluate the utility of transdermal fentanyl (TDF, Durogesic) for the treatment of pain due to osteoarthritis (OA) of the knee or hip, which was not adequately controlled by non-opioid analgesics or weak opioids. The second part of the trial, investigating TDF in patients with rheumatoid arthritis (RA) is reported separately.
Methods: Current analgesia was optimised during a 1-week run-in. Patients then received 28 days treatment with TDF starting at 25 microg/hr, with the option to increase the dose until adequate pain control was achieved. Metoclopramide was taken during the first week and then as needed.
Results: Of the 159 patients recruited, 75 with OA knee and 44 with OA hip completed the treatment phase, 30 knee and 18 hip patients entered the one-week taper-off phase. The most frequently used maximum dose of TDF was 25 microg/hr. The number of patients with adequate pain control increased during the run-in period from 4% to 27%, and further increased during TDF treatment to 88% on day 28. From baseline to endpoint, there were significant reductions in pain (p < 0.001) and improvements in functioning (p < 0.001) and physical (p < 0.001) and mental (p < 0.05) health. Scores for 'pain right now' decreased significantly within 24 hours of starting TDF treatment. TDF was assessed favourably and 84% of patients would recommend it for OA-related pain. Nausea and vomiting were the most common adverse events (reported by 32% and 26% of patients respectively), despite prophylaxis with metoclopramide, which showed limited efficacy in this setting.
Conclusion: TDF significantly increased pain control, and improved functioning and quality of life. Metoclopramide appeared to be of limited value in preventing nausea and vomiting; more effective anti-emetic treatment may enable more people to benefit from strong opioids such as TDF. This study suggests that four weeks is a reasonable period to test the benefit of adding TDF to improve pain control in OA patients and that discontinuing therapy in cases of limited benefit creates no major obstacles.
Figures
References
-
- Arthritis Research Campaign Factfile – arthritis at a glance. 2003. http://http.//www.arc.org.uk
-
- Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clin Geriatr Med. 1998;14:435–454. - PubMed
-
- Pham T, van der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. Outcome variables for osteoarthritis clinical trials: The OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648–1654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical