Genetic, genomic, and functional analysis of the granule lattice proteins in Tetrahymena secretory granules
- PMID: 15958493
- PMCID: PMC1196318
- DOI: 10.1091/mbc.e05-01-0028
Genetic, genomic, and functional analysis of the granule lattice proteins in Tetrahymena secretory granules
Abstract
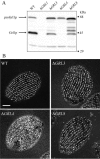
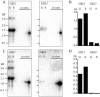
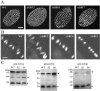
In some cells, the polypeptides stored in dense core secretory granules condense as ordered arrays. In ciliates such as Tetrahymena thermophila, the resulting crystals function as projectiles, expanding upon exocytosis. Isolation of granule contents previously defined five Granule lattice (Grl) proteins as abundant core constituents, whereas a functional screen identified a sixth family member. We have now expanded this screen to identify the nonredundant components required for projectile assembly. The results, further supported by gene disruption experiments, indicate that six Grl proteins define the core structure. Both in vivo and in vitro data indicate that core assembly begins in the endoplasmic reticulum with formation of specific hetero-oligomeric Grl proprotein complexes. Four additional GRL-like genes were found in the T. thermophila genome. Grl2p and Grl6p are targeted to granules, but the transcripts are present at low levels and neither is essential for core assembly. The DeltaGRL6 cells nonetheless showed a subtle change in granule morphology and a marked reduction in granule accumulation. Epistasis analysis suggests this results from accelerated loss of DeltaGRL6 granules, rather than from decreased synthesis. Our results not only provide insight into the organization of Grl-based granule cores but also imply that the functions of Grl proteins extend beyond core assembly.
Figures








Similar articles
-
Independent transport and sorting of functionally distinct protein families in Tetrahymena thermophila dense core secretory granules.Eukaryot Cell. 2009 Oct;8(10):1575-83. doi: 10.1128/EC.00151-09. Epub 2009 Aug 14. Eukaryot Cell. 2009. PMID: 19684282 Free PMC article.
-
New class of cargo protein in Tetrahymena thermophila dense core secretory granules.Eukaryot Cell. 2002 Aug;1(4):583-93. doi: 10.1128/EC.1.4.583-593.2002. Eukaryot Cell. 2002. PMID: 12456006 Free PMC article.
-
Granule lattice protein 1 (Grl1p), an acidic, calcium-binding protein in Tetrahymena thermophila dense-core secretory granules, influences granule size, shape, content organization, and release but not protein sorting or condensation.J Cell Biol. 1996 Dec;135(6 Pt 2):1775-87. doi: 10.1083/jcb.135.6.1775. J Cell Biol. 1996. PMID: 8991090 Free PMC article.
-
Out with a bang! Tetrahymena as a model system to study secretory granule biogenesis.Traffic. 2004 Feb;5(2):63-8. doi: 10.1046/j.1600-0854.2003.00155.x. Traffic. 2004. PMID: 14690495 Review.
-
Secretory granule exocytosis.Physiol Rev. 2003 Apr;83(2):581-632. doi: 10.1152/physrev.00031.2002. Physiol Rev. 2003. PMID: 12663867 Review.
Cited by
-
The Co-regulation Data Harvester: automating gene annotation starting from a transcriptome database.SoftwareX. 2017;6:165-171. doi: 10.1016/j.softx.2017.06.006. Epub 2017 Aug 16. SoftwareX. 2017. PMID: 29104906 Free PMC article.
-
Independent transport and sorting of functionally distinct protein families in Tetrahymena thermophila dense core secretory granules.Eukaryot Cell. 2009 Oct;8(10):1575-83. doi: 10.1128/EC.00151-09. Epub 2009 Aug 14. Eukaryot Cell. 2009. PMID: 19684282 Free PMC article.
-
Functional GFP-metallothionein fusion protein from Tetrahymena thermophila: a potential whole-cell biosensor for monitoring heavy metal pollution and a cell model to study metallothionein overproduction effects.Biometals. 2014 Feb;27(1):195-205. doi: 10.1007/s10534-014-9704-0. Epub 2014 Jan 16. Biometals. 2014. PMID: 24430977 Free PMC article.
-
An endosomal syntaxin and the AP-3 complex are required for formation and maturation of candidate lysosome-related secretory organelles (mucocysts) in Tetrahymena thermophila.Mol Biol Cell. 2017 Jun 1;28(11):1551-1564. doi: 10.1091/mbc.E17-01-0018. Epub 2017 Apr 5. Mol Biol Cell. 2017. PMID: 28381425 Free PMC article.
-
An apical membrane complex for triggering rhoptry exocytosis and invasion in Toxoplasma.EMBO J. 2022 Nov 17;41(22):e111158. doi: 10.15252/embj.2022111158. Epub 2022 Oct 17. EMBO J. 2022. PMID: 36245278 Free PMC article.
References
-
- Adoutte, A., Garreau de Loubresse, N., and Beisson, J. (1984). Proteolytic cleavage and maturation of the crystalline secretion products of Paramecium. J. Mol. Biol. 180, 1065–1081. - PubMed
-
- Andres, D. A., Rhodes, J. D., Meisel, R. L., and Dixon, J. E. (1991). Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J. Biol. Chem. 266, 14277–14282. - PubMed
-
- Arvan, P., and Castle, J. D. (1992). Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell. Biol. 2, 327–331. - PubMed
-
- Arvan, P., Zhang, B. Y., Feng, L., Liu, M., and Kuliawat, R. (2002). Lumenal protein multimerization in the distal secretory pathway/secretory granules. Curr. Opin. Cell Biol. 14, 448–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

