Regulation of lck degradation and refractory state in CD8+ cytotoxic T lymphocytes
- PMID: 15958529
- PMCID: PMC1166584
- DOI: 10.1073/pnas.0406333102
Regulation of lck degradation and refractory state in CD8+ cytotoxic T lymphocytes
Abstract
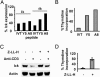
After specific activation, CD8+ cytotoxic T lymphocytes (CTLs) enter a refractory state termed activation-induced nonresponsiveness (AINR) that is characterized by the inability of T cells to respond to a secondary stimulus. Here, we show that T cell receptor triggering results in rapid degradation of the src-family protein kinase lck through a mechanism that is proteasome- and lysosome-independent, sensitive to cysteine protease inhibitors, and distinct from the pathways involved in degradation of ZAP-70 kinase or zeta-chain of the CD3 complex. Pharmacologic blockade of lck degradation, as well as transfection of refractory cells with an lck expression vector, increased responsiveness of CTLs to repeated antigenic challenge. The development or maintenance of AINR was not affected by exogenously added IL-2, whereas IL-15 or IFN-alpha restored both lck expression and responsiveness of preactivated CTLs. Our results suggest that lck degradation plays an important role in the development of AINR in human CTLs and that this condition can be reverted by pharmacologic agents or lymphokines that prevent lck degradation or induce its expression.
Figures
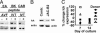




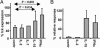
Similar articles
-
Deficient expression of p56(lck) in Th2 cells leads to partial TCR signaling and a dysregulation in lymphokine mRNA levels.J Immunol. 1996 Dec 1;157(11):4751-61. J Immunol. 1996. PMID: 8943376
-
The p56lck SH2 domain mediates recruitment of CD8/p56lck to the activated T cell receptor/CD3/zeta complex.Eur J Immunol. 1996 Sep;26(9):2093-2100. doi: 10.1002/eji.1830260920. Eur J Immunol. 1996. PMID: 8814252
-
Activation of T cells via CD55: recruitment of early components of the CD3-TCR pathway is required for IL-2 secretion.J Inflamm. 1998;48(1):13-27. J Inflamm. 1998. PMID: 9368189
-
Endocytic regulation of the T lymphocyte co-receptor proteins CD4 and CD8.Biochem Soc Trans. 1993 Aug;21 ( Pt 3)(3):703-6. doi: 10.1042/bst0210703. Biochem Soc Trans. 1993. PMID: 8224493 Review. No abstract available.
-
Lck Function and Modulation: Immune Cytotoxic Response and Tumor Treatment More Than a Simple Event.Cancers (Basel). 2024 Jul 24;16(15):2630. doi: 10.3390/cancers16152630. Cancers (Basel). 2024. PMID: 39123358 Free PMC article. Review.
Cited by
-
Characterization of long-term mixed donor-donor chimerism after double cord blood transplantation.Clin Exp Immunol. 2010 Oct;162(1):146-55. doi: 10.1111/j.1365-2249.2010.04212.x. Epub 2010 Aug 20. Clin Exp Immunol. 2010. PMID: 20731674 Free PMC article.
-
Combining Flow and Mass Cytometry in the Search for Biomarkers in Chronic Graft-versus-Host Disease.Front Immunol. 2017 Jun 19;8:717. doi: 10.3389/fimmu.2017.00717. eCollection 2017. Front Immunol. 2017. PMID: 28674539 Free PMC article.
-
Expansion of Gammadelta T Cells from Cord Blood: A Therapeutical Possibility.Stem Cells Int. 2018 Mar 7;2018:8529104. doi: 10.1155/2018/8529104. eCollection 2018. Stem Cells Int. 2018. PMID: 29707004 Free PMC article.
-
Long-Term Stable Mixed Chimerism after Hematopoietic Stem Cell Transplantation in Patients with Non-Malignant Disease, Shall We Be Tolerant?PLoS One. 2016 May 6;11(5):e0154737. doi: 10.1371/journal.pone.0154737. eCollection 2016. PLoS One. 2016. PMID: 27152621 Free PMC article.
-
Molecular basis for checkpoints in the CD8 T cell response: tolerance versus activation.Semin Immunol. 2007 Jun;19(3):153-61. doi: 10.1016/j.smim.2007.02.007. Epub 2007 Mar 26. Semin Immunol. 2007. PMID: 17382557 Free PMC article. Review.
References
-
- Höllsberg, P., Batra, V., Dressel, A. & Hafler, D. A. (1996) J. Immunol. 157, 5269-5276. - PubMed
-
- Deeths, M. J., Kedl, R. M. & Mescher, M. F. (1999) J. Immunol. 163, 102-110. - PubMed
-
- Tham, E. L. & Mescher, M. F. (2002) J. Immunol. 169, 1822-1828. - PubMed
-
- Heissmeyer, V., Macian, F., Im, S. H., Varma, R., Feske, S., Venuprasad, K., Gu, H., Liu, Y. C., Dustin, M. L. & Rao, A. (2004) Nat. Immunol. 5, 255-265. - PubMed
-
- Tham, E. L. & Mescher, M. F. (2001) J. Immunol. 167, 2040-2048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

