Validation of genomics-based prognostic tests in malignant pleural mesothelioma
- PMID: 15958625
- PMCID: PMC1488818
- DOI: 10.1158/1078-0432.CCR-04-2181
Validation of genomics-based prognostic tests in malignant pleural mesothelioma
Abstract
Purpose: Malignant pleural mesothelioma (MPM) is a highly lethal neoplasm with limited pretreatment prognostication strategies. In this report, we examine the accuracy of a previously proposed prognostic test in an independent cohort of MPM patients. This test uses simple ratios of gene expression levels to provide a novel prognostication scheme.
Experimental design: Gene expression data using high-density oligonucleotide microarrays (approximately 22,000 genes) were obtained for a new cohort of human MPM tumors from patients undergoing similar treatments (n = 39). The relative expression levels for specific genes were also determined using real-time quantitative reverse transcription-PCR. We also used a subset of these tumors associated with widely divergent patient survival (n = 23) as a training set to identify new treatment-specific candidate prognostic molecular markers and gene ratio-based prognostic tests. The predictive nature of these newly discovered markers and gene ratio-based prognostic tests were then examined in an independent group of tumors (n = 52) using microarray data and quantitative reverse transcription-PCR.
Results: Previously described MPM prognostic genes and gene ratio-based prognostic tests predicted clinical outcome in 39 independent MPM tumor specimens in a statistically significant manner. Newly discovered treatment-specific prognostic genes and gene ratio-based prognostic tests were highly accurate and statistically significant when examined in an independent group of 52 tumors from patients undergoing similar treatment.
Conclusions: The data support the use of gene ratios in translating gene expression data into easily reproducible, statistically validated clinical tests for the prediction of outcome in MPM.
Figures
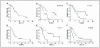
References
-
- Pass H. Malignant pleural mesothelioma: surgical roles and novel therapies. Clin Lung Cancer. 2001;3:102–17. Medline. - PubMed
-
- Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–9. Medline. - PubMed
-
- Sugarbaker DJ, Liptay MJ. Therapeutic approaches in malignant mesothelioma. In: Aisner J, Arriagada R, Green MR, Martini N, Perry MC, editors. Comprehensive textbook of thoracic oncology. Williams and Wilkins; Baltimore (MD): 1996. pp. 786–98.
-
- Aisner J. Diagnosis, staging, and natural history of pleural mesothelioma. In: Aisner J, Arriagada R, Green MR, Martini N, Perry MC, editors. Comprehensive textbook of thoracic oncology. Williams and Wilkins; Baltimore (MD): 1996. pp. 799–85.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

