Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat
- PMID: 15958736
- PMCID: PMC6724886
- DOI: 10.1523/JNEUROSCI.0523-05.2005
Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat
Abstract
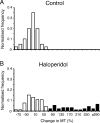
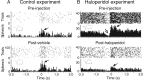
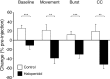
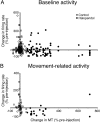
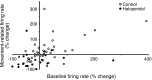
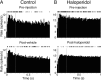
Disruption of motor cortex activity is hypothesized to play a major role in the slowed movement (bradykinesia) associated with reduced dopaminergic function. We recorded single neurons in the motor cortex of free-moving rats performing a forelimb-reaching task. The same neurons were examined before and after induction of bradykinesia with the D2 dopamine receptor antagonist haloperidol. Within-cell changes in the firing rate and firing pattern of individual cells and the correlation between simultaneously recorded cells after injection of haloperidol were statistically compared with vehicle-only control experiments. During haloperidol-induced bradykinesia (mean movement time increase, +231%), there was an average 11% decrease in baseline firing rate. Movement-related peaks in firing rate were more dramatically affected, with an overall reduction in peak amplitudes of 40%. Bradykinesia was also associated with decreased intensity of bursting and amplitude of cross-correlation peaks at rest. The results show for the first time that significant reductions can be detected in motor cortex activity at rest in animals with impaired ability to generate movements induced by reduced dopamine action and confirm that impaired movements are associated with reduced cortical activation. Together, these changes in neural activity may reduce recruitment and rate modulation of motor units in the spinal cord.
Figures

References
-
- Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366-375. - PubMed
-
- Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266-271. - PubMed
-
- Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson's disease. Brain 124: 2131-2146. - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR (1994) The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507-520. - PubMed
-
- Bonfiglioli C, DeBerti G, Nichelli P, Nicoletti R, Castiello U (1998) Kinematic analysis of the reach to grasp movement in Parkinson's and Huntington's disease subjects. Neuropsychologia 36: 1203-1208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
