Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired-associate memory
- PMID: 15958738
- PMCID: PMC6724878
- DOI: 10.1523/JNEUROSCI.4935-04.2005
Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired-associate memory
Abstract
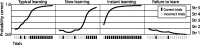
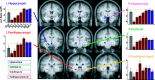
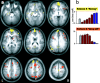
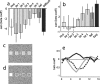
Recent neurophysiological findings from the monkey hippocampus showed dramatic changes in the firing rate of individual hippocampal cells as a function of learning new associations. To extend these findings to humans, we used blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to examine the patterns of brain activity during learning of an analogous associative task. We observed bilateral, monotonic increases in activity during learning not only in the hippocampus but also in the parahippocampal and right perirhinal cortices. In addition, activity related to simple novelty signals was observed throughout the medial temporal lobe (MTL) memory system and in several frontal regions. A contrasting pattern was observed in a frontoparietal network in which a high level of activity was sustained until the association was well learned, at which point the activity decreased to baseline. Thus, we found that associative learning in humans is accompanied by striking increases in BOLD fMRI activity throughout the MTL as well as in the cingulate cortex and frontal lobe, consistent with neurophysiological findings in the monkey hippocampus. The finding that both the hippocampus and surrounding MTL cortex exhibited similar associative learning and novelty signals argues strongly against the view that there is a clear division of labor in the MTL in which the hippocampus is essential for forming associations and the cortex is involved in novelty detection. A second experiment addressed a striking aspect of the data from the first experiment by demonstrating a substantial effect of baseline task difficulty on MTL activity capable of rendering mnemonic activity as either "positive" or "negative."
Figures
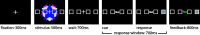
References
-
- Aggleton JP, Brown MW (1999) Episodic memory, amnesia, and the hippocampal-thalamic axis. Behav Brain Sci 22: 425-444. - PubMed
-
- Brown MW, Aggleton JP (2001) Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51-61. - PubMed
-
- Cabeza R, Nyberg L (2000) Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1-47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
