Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system
- PMID: 15958739
- PMCID: PMC6724884
- DOI: 10.1523/JNEUROSCI.0096-05.2005
Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system
Erratum in
- J Neurosci. 2005 Jun 29;25(26):2 p following 6262
Abstract
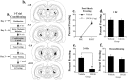
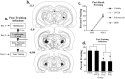
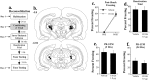
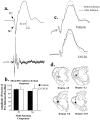
In the present study, we examined the role of the auditory thalamus [medial division of the medial geniculate nucleus and the adjacent posterior intralaminar nucleus (MGm/PIN)] in auditory pavlovian fear conditioning using pharmacological manipulation of intracellular signaling pathways. In the first experiment, rats were given intrathalamic infusions of the MEK (mitogen-activated protein kinase-kinase) inhibitor 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene (U0126) before fear conditioning. Findings revealed that long-term memory (assessed at 24 h) was impaired, whereas short-term memory (assessed at 1-3 h) of fear conditioning was intact. In the second experiment, rats received immediate posttraining intrathalamic infusion of U0126, the mRNA synthesis inhibitor 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB), or infusion of the protein synthesis inhibitor anisomycin. Posttraining infusion of either U0126 or DRB significantly impaired long-term retention of fear conditioning, whereas infusion of anisomycin had no effect. In the final experiment, rats received intrathalamic infusion of U0126 before long-term potentiation (LTP)-inducing stimulation of thalamic inputs to the lateral nucleus of the amygdala (LA). Findings revealed that thalamic infusion of U0126 impaired LTP in the LA. Together, these results suggest the possibility that MGm/PIN cells that project to the LA contribute to memory formation via ERK (extracellular signal-regulated kinase)-mediated transcription, but that they do so by promoting protein synthesis-dependent plasticity locally in the LA.
Figures


References
-
- Antonov I, Antonova I, Kandel ER, Hawkins RD (2003) Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia Neuron 37: 135-147. - PubMed
-
- Arancio O, Kandel ER, Hawkins RD (1995) Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature 376: 74-80. - PubMed
-
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD (1996) Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 87: 1025-1035. - PubMed
-
- Bailey CH, Kandel ER (1993) Structural changes accompanying memory storage. Annu Rev Physiol 55: 397-426. - PubMed
-
- Bailey CH, Montarolo P, Chen M, Kandel ER, Schacher S (1992) Inhibitors of protein and RNA synthesis block structural changes that accompany long-term heterosynaptic plasticity in Aplysia Neuron 9: 749-758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
