Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly
- PMID: 15959508
- PMCID: PMC1386036
- DOI: 10.1038/nature03606
Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly
Abstract
The atomic structure of tubulin in a polymerized, straight protofilament is clearly distinct from that in a curved conformation bound to a cellular depolymerizer. The nucleotide contents are identical, and in both cases the conformation of the GTP-containing, intra-dimer interface is indistinguishable from the GDP-containing, inter-dimer contact. Here we present two structures corresponding to the start and end points in the microtubule polymerization and hydrolysis cycles that illustrate the consequences of nucleotide state on longitudinal and lateral assembly. In the absence of depolymerizers, GDP-bound tubulin shows distinctive intra-dimer and inter-dimer interactions and thus distinguishes the GTP and GDP interfaces. A cold-stable tubulin polymer with the non-hydrolysable GTP analogue GMPCPP, containing semi-conserved lateral interactions, supports a model in which the straightening of longitudinal interfaces happens sequentially, starting with a conformational change after GTP binding that straightens the dimer enough for the formation of lateral contacts into a non-tubular intermediate. Closure into a microtubule does not require GTP hydrolysis.
Conflict of interest statement
The authors have no financial interest concerning this work.
Figures


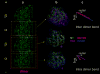
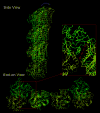


Comment in
-
Cell biology: powerful curves.Nature. 2005 Jun 16;435(7044):895-7. doi: 10.1038/435895a. Nature. 2005. PMID: 15959501 No abstract available.
Similar articles
-
Thermodynamic and structural analysis of microtubule assembly: the role of GTP hydrolysis.Biophys J. 1997 Mar;72(3):1357-75. doi: 10.1016/S0006-3495(97)78782-4. Biophys J. 1997. PMID: 9138581 Free PMC article.
-
Intrinsic bending of microtubule protofilaments.Structure. 2011 Mar 9;19(3):409-17. doi: 10.1016/j.str.2010.12.020. Structure. 2011. PMID: 21397191
-
Mechanical properties of tubulin intra- and inter-dimer interfaces and their implications for microtubule dynamic instability.PLoS Comput Biol. 2019 Aug 30;15(8):e1007327. doi: 10.1371/journal.pcbi.1007327. eCollection 2019 Aug. PLoS Comput Biol. 2019. PMID: 31469822 Free PMC article.
-
Tubulin rings: which way do they curve?Curr Opin Struct Biol. 2003 Apr;13(2):256-61. doi: 10.1016/s0959-440x(03)00029-0. Curr Opin Struct Biol. 2003. PMID: 12727521 Review.
-
Guanosine-5'-triphosphate hydrolysis and tubulin polymerization. Review article.Mol Cell Biochem. 1982 Sep 3;47(2):97-113. doi: 10.1007/BF00234410. Mol Cell Biochem. 1982. PMID: 6755216 Review.
Cited by
-
Microtubule-associated proteins control the kinetics of microtubule nucleation.Nat Cell Biol. 2015 Jul;17(7):907-16. doi: 10.1038/ncb3188. Epub 2015 Jun 22. Nat Cell Biol. 2015. PMID: 26098575
-
The state of the guanosine nucleotide allosterically affects the interfaces of tubulin in protofilament.J Comput Aided Mol Des. 2012 Apr;26(4):397-407. doi: 10.1007/s10822-012-9566-x. Epub 2012 Apr 19. J Comput Aided Mol Des. 2012. PMID: 22527959
-
The Ndc80 kinetochore complex directly modulates microtubule dynamics.Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):16113-8. doi: 10.1073/pnas.1209615109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908300 Free PMC article.
-
GDP-tubulin incorporation into growing microtubules modulates polymer stability.J Biol Chem. 2010 Jun 4;285(23):17507-13. doi: 10.1074/jbc.M109.099515. Epub 2010 Apr 6. J Biol Chem. 2010. PMID: 20371874 Free PMC article.
-
Drosophila as a genetic and cellular model for studies on axonal growth.Neural Dev. 2007 May 2;2:9. doi: 10.1186/1749-8104-2-9. Neural Dev. 2007. PMID: 17475018 Free PMC article. Review.
References
-
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. - PubMed
-
- Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anti-Canc Agents. 2002;2:1–17. - PubMed
-
- Heald R, Nogales E. Microtubule dynamics. J Cell Sci. 2002;115:3–4. - PubMed
-
- Nogales E, Wolf SG, Downing KH. Structure of the αβ tubulin dimer by electron crystallography. Nature. 1998;391:199–203. - PubMed
-
- Löwe J, Li H, Downing KH, Nogales E. Refined structure of αβ-tubulin at 3.5 Å resolution. J Mol Biol. 2001;313:1045–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

