Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools
- PMID: 15960610
- PMCID: PMC1237135
- DOI: 10.1042/BJ20050342
Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools
Abstract
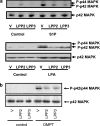
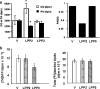
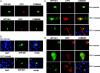
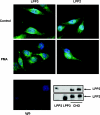
We have shown previously that LPPs (lipid phosphate phosphatases) reduce the stimulation of the p42/p44 MAPK (p42/p44 mitogen-activated protein kinase) pathway by the GPCR (G-protein-coupled receptor) agonists S1P (sphingosine 1-phosphate) and LPA (lysophosphatidic acid) in serum-deprived HEK-293 cells [Alderton, Darroch, Sambi, McKie, Ahmed, N. J. Pyne and S. Pyne (2001) J. Biol. Chem. 276, 13452-13460]. In the present study, we now show that this can be blocked by pretreating HEK-293 cells with the caspase 3/7 inhibitor, Ac-DEVD-CHO [N-acetyl-Asp-Glu-Val-Asp-CHO (aldehyde)]. Therefore LPP2 and LPP3 appear to regulate the apoptotic status of serum-deprived HEK-293 cells. This was supported further by: (i) caspase 3/7-catalysed cleavage of PARP [poly(ADP-ribose) polymerase] was increased in serum-deprived LPP2-overexpressing compared with vector-transfected HEK-293 cells; and (ii) serum-deprived LPP2- and LPP3-overexpressing cells exhibited limited intranucleosomal DNA laddering, which was absent in vector-transfected cells. Moreover, LPP2 reduced basal intracellular phosphatidic acid levels, whereas LPP3 decreased intracellular S1P in serum-deprived HEK-293 cells. LPP2 and LPP3 are constitutively co-localized with SK1 (sphingosine kinase 1) in cytoplasmic vesicles in HEK-293 cells. Moreover, LPP2 but not LPP3 prevents SK1 from being recruited to a perinuclear compartment upon induction of PLD1 (phospholipase D1) in CHO (Chinese-hamster ovary) cells. Taken together, these data are consistent with an important role for LPP2 and LPP3 in regulating an intracellular pool of PA and S1P respectively, that may govern the apoptotic status of the cell upon serum deprivation.
Figures

References
-
- Brindley D. N., Waggoner D. W. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 1998;273:24281–24284. - PubMed
-
- Kai M., Wada I., Imai S., Sakane F., Kanoh H. Identification and cDNA cloning of 35-kDa phosphatidic acid phosphatase (type 2) bound to plasma membranes. Polymerase chain reaction amplification of mouse H2O2-inducible hic53 clone yielded the cDNA encoding phosphatidic acid phosphatase. J. Biol. Chem. 1996;271:18931–18938. - PubMed
-
- Kai M., Wada I., Imai S., Sakane F., Kanoh H. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J. Biol. Chem. 1997;272:24572–24578. - PubMed
-
- Roberts R., Sciorra V. A., Morris A. J. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 1998;273:22059–22067. - PubMed
-
- Leung D. W., Tompkins C. K., White T. Molecular cloning of two alternatively spliced forms of human phosphatidic acid phosphatase cDNAs that are differentially expressed in normal and tumor cells. DNA Cell. Biol. 1998;17:377–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

