Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala
- PMID: 1596084
- PMCID: PMC3153947
- DOI: 10.1002/ana.410310505
Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala
Abstract
The neuroanatomical substrate of seizures induced by picomolar amounts of corticotropin-releasing hormone in infant rats was investigated. Electrographic and behavioral phenomena were monitored in 42 rat pups aged 5 to 22 days. Rat pups carried bipolar electrodes implanted in subcortical limbic structures, as well as cortical electrodes and intracerebroventricular cannulae. The administration of corticotropin-releasing hormone produced age-specific seizures within minutes, which correlated with rhythmic amygdala discharges. Paroxysmal hippocampal and cortical discharges developed subsequently in some rats. Corticotropin-releasing hormone-induced electrographic and behavioral seizures originate in the amygdala.
Figures

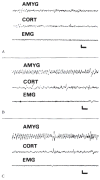


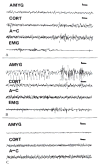
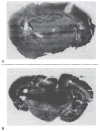
References
-
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. - PubMed
-
- Vale W, Rivier C, Brown MR, et al. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;83:245–270. - PubMed
-
- Koob GF, Britton KT. Behavioral effects of corticotropin-releasing factor. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC; 1990. pp. 253–265.
-
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin releasing factor activates neurons of the locus coeruleus. Brain Res. 1983;270:363–367. - PubMed
-
- Siggins GR. Electrophysiology of corticotropin-releasing factor in nervous tissue. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC; 1990. pp. 205–214.

