The involvement of Cav3.2/alpha1H T-type calcium channels in excitability of mouse embryonic primary vestibular neurones
- PMID: 15961427
- PMCID: PMC1474151
- DOI: 10.1113/jphysiol.2005.089342
The involvement of Cav3.2/alpha1H T-type calcium channels in excitability of mouse embryonic primary vestibular neurones
Abstract
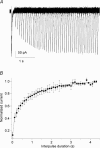
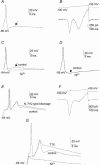
Ca2+ influx through voltage-gated calcium channels probably influences neuronal ontogenesis. Many developing neurones transiently express T-type/Cav3 calcium channels that contribute to their electrical activity and potentially to their morphological differentiation. Here we have characterized the electrophysiological properties and the functional role of a large T-type calcium current that is present in mouse developing primary vestibular neurones at embryonic day E17. This T-type current showed fast activation and inactivation, as well as slow deactivation kinetics. The overlap of activation and inactivation parameters produced a window current between -65 and -45 mV. Recovery from short-term inactivation was slow suggesting the presence of the Cav3.2 subunit. This T-type current was blocked by micromolar concentrations of Ni2+ and was inhibited by fast perfusion velocities in a similar fashion to recombinant Cav3.2 T-type channels expressed in HEK-293 cells. More importantly, current clamp experiments have revealed that the T-current could elicit afterdepolarization potentials during the repolarization phase of action potentials, and occasionally generate calcium spikes. Taken together, we demonstrate that the Cav3.2 subunit is likely to be the main T-type calcium channel subunit expressed in embryonic vestibular neurones and should play a key role in the excitability of these neurones during the ontogenesis of vestibular afferentation.
Figures




References
-
- Anniko M. Early development and maturation of the spiral ganglion. Acta Otolaryngol. 1983;95:263–276. - PubMed
-
- Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

