Kir6.2 mutations causing neonatal diabetes provide new insights into Kir6.2-SUR1 interactions
- PMID: 15962003
- PMCID: PMC1173155
- DOI: 10.1038/sj.emboj.7600715
Kir6.2 mutations causing neonatal diabetes provide new insights into Kir6.2-SUR1 interactions
Abstract
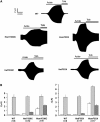
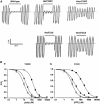
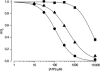
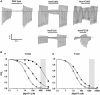
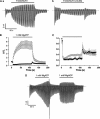
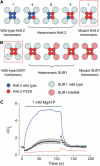
ATP-sensitive K(+) (K(ATP)) channels, comprised of pore-forming Kir6.2 and regulatory SUR1 subunits, play a critical role in regulating insulin secretion. Binding of ATP to Kir6.2 inhibits, whereas interaction of MgATP with SUR1 activates, K(ATP) channels. We tested the functional effects of two Kir6.2 mutations (Y330C, F333I) that cause permanent neonatal diabetes mellitus, by heterologous expression in Xenopus oocytes. Both mutations reduced ATP inhibition and increased whole-cell currents, which in pancreatic beta-cells is expected to reduce insulin secretion and precipitate diabetes. The Y330C mutation reduced ATP inhibition both directly, by impairing ATP binding (and/or transduction), and indirectly, by stabilizing the intrinsic open state of the channel. The F333I mutation altered ATP binding/transduction directly. Both mutations also altered Kir6.2/SUR1 interactions, enhancing the stimulatory effect of MgATP (which is mediated via SUR1). This effect was particularly dramatic for the Kir6.2-F333I mutation, and was abolished by SUR1 mutations that prevent MgATP binding/hydrolysis. Further analysis of F333I heterozygous channels indicated that at least three SUR1 must bind/hydrolyse MgATP to open the mutant K(ATP) channel.
Figures

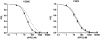
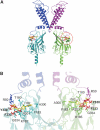
Similar articles
-
Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes.Pflugers Arch. 2006 Dec;453(3):323-32. doi: 10.1007/s00424-006-0112-3. Epub 2006 Sep 22. Pflugers Arch. 2006. PMID: 17021801
-
The Kir6.2-F333I mutation differentially modulates KATP channels composed of SUR1 or SUR2 subunits.J Physiol. 2007 Jun 15;581(Pt 3):1259-69. doi: 10.1113/jphysiol.2007.130211. Epub 2007 Mar 29. J Physiol. 2007. PMID: 17395632 Free PMC article.
-
Functional effects of naturally occurring KCNJ11 mutations causing neonatal diabetes on cloned cardiac KATP channels.J Physiol. 2006 Feb 15;571(Pt 1):3-14. doi: 10.1113/jphysiol.2005.099168. Epub 2005 Dec 8. J Physiol. 2006. PMID: 16339180 Free PMC article.
-
Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):257-67. doi: 10.1098/rstb.2008.0142. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18990670 Free PMC article. Review.
-
Diabetes and hypoglycaemia in young children and mutations in the Kir6.2 subunit of the potassium channel: therapeutic consequences.Diabetes Metab. 2006 Dec;32(6):569-80. doi: 10.1016/S1262-3636(07)70311-7. Diabetes Metab. 2006. PMID: 17296510 Review.
Cited by
-
An in-frame deletion in Kir6.2 (KCNJ11) causing neonatal diabetes reveals a site of interaction between Kir6.2 and SUR1.J Clin Endocrinol Metab. 2009 Jul;94(7):2551-7. doi: 10.1210/jc.2009-0159. Epub 2009 Apr 7. J Clin Endocrinol Metab. 2009. PMID: 19351728 Free PMC article.
-
Incomplete dissociation of glibenclamide from wild-type and mutant pancreatic K ATP channels limits their recovery from inhibition.Br J Pharmacol. 2009 Jan;156(2):354-61. doi: 10.1111/j.1476-5381.2008.00005.x. Epub 2009 Jan 13. Br J Pharmacol. 2009. PMID: 19154434 Free PMC article.
-
LKB1 couples glucose metabolism to insulin secretion in mice.Diabetologia. 2015 Jul;58(7):1513-22. doi: 10.1007/s00125-015-3579-7. Epub 2015 Apr 16. Diabetologia. 2015. PMID: 25874445
-
Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes.Pflugers Arch. 2006 Dec;453(3):323-32. doi: 10.1007/s00424-006-0112-3. Epub 2006 Sep 22. Pflugers Arch. 2006. PMID: 17021801
-
Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle.J Am Heart Assoc. 2013 Aug 23;2(4):e000365. doi: 10.1161/JAHA.113.000365. J Am Heart Assoc. 2013. PMID: 23974906 Free PMC article.
References
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement IV JP, Boyd AE III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA (1995) Cloning of the β-cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268: 423–426 - PubMed
-
- Ashcroft FM, Gribble FM (1998) Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci 21: 288–294 - PubMed
-
- Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic β-cell. Prog Biophys Mol Biol 54: 87–143 - PubMed
-
- Ashcroft FM, Rorsman P (2004) Type-2 diabetes mellitus: not quite exciting enough? Hum Mol Genet 13: R21–R31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

