VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis
- PMID: 15962004
- PMCID: PMC1173150
- DOI: 10.1038/sj.emboj.7600709
VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis
Abstract
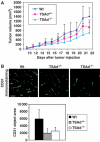
Vascular endothelial growth factor receptor-2 (VEGFR-2) activation by VEGF-A is essential in vasculogenesis and angiogenesis. We have generated a pan-phosphorylation site map of VEGFR-2 and identified one major tyrosine phosphorylation site in the kinase insert (Y951), in addition to two major sites in the C-terminal tail (Y1175 and Y1214). In developing vessels, phosphorylation of Y1175 and Y1214 was detected in all VEGFR-2-expressing endothelial cells, whereas phosphorylation of Y951 was identified in a subset of vessels. Phosphorylated Y951 bound the T-cell-specific adapter (TSAd), which was expressed in tumor vessels. Mutation of Y951 to F and introduction of phosphorylated Y951 peptide or TSAd siRNA into endothelial cells blocked VEGF-A-induced actin stress fibers and migration, but not mitogenesis. Tumor vascularization and growth was reduced in TSAd-deficient mice, indicating a critical role of Y951-TSAd signaling in pathological angiogenesis.
Figures




Similar articles
-
Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis.J Atheroscler Thromb. 2006 Jun;13(3):130-5. doi: 10.5551/jat.13.130. J Atheroscler Thromb. 2006. PMID: 16835467 Review.
-
VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd.J Exp Med. 2012 Jul 2;209(7):1363-77. doi: 10.1084/jem.20111343. Epub 2012 Jun 11. J Exp Med. 2012. PMID: 22689825 Free PMC article.
-
The endothelial adaptor molecule TSAd is required for VEGF-induced angiogenic sprouting through junctional c-Src activation.Sci Signal. 2016 Jul 19;9(437):ra72. doi: 10.1126/scisignal.aad9256. Sci Signal. 2016. PMID: 27436360
-
Functional and structural characterization of the kinase insert and the carboxy terminal domain in VEGF receptor 2 activation.FASEB J. 2014 Nov;28(11):4914-23. doi: 10.1096/fj.14-256206. Epub 2014 Aug 11. FASEB J. 2014. PMID: 25114179
-
Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials.Exp Eye Res. 2006 Nov;83(5):1005-16. doi: 10.1016/j.exer.2006.03.019. Epub 2006 May 19. Exp Eye Res. 2006. PMID: 16713597 Free PMC article. Review.
Cited by
-
Protein post-translational modifications in the regulation of cancer hallmarks.Cancer Gene Ther. 2023 Apr;30(4):529-547. doi: 10.1038/s41417-022-00464-3. Epub 2022 Apr 7. Cancer Gene Ther. 2023. PMID: 35393571 Review.
-
Interaction between BEND5 and RBPJ suppresses breast cancer growth and metastasis via inhibiting Notch signaling.Int J Biol Sci. 2022 Jun 27;18(10):4233-4244. doi: 10.7150/ijbs.70866. eCollection 2022. Int J Biol Sci. 2022. PMID: 35844785 Free PMC article.
-
Mechanoregulation of Vascular Endothelial Growth Factor Receptor 2 in Angiogenesis.Front Cardiovasc Med. 2022 Jan 11;8:804934. doi: 10.3389/fcvm.2021.804934. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35087885 Free PMC article. Review.
-
The cellular response to vascular endothelial growth factors requires co-ordinated signal transduction, trafficking and proteolysis.Biosci Rep. 2015 Aug 18;35(5):e00253. doi: 10.1042/BSR20150171. Biosci Rep. 2015. PMID: 26285805 Free PMC article. Review.
-
Disorders of Vascular Permeability.Annu Rev Pathol. 2016 May 23;11:251-81. doi: 10.1146/annurev-pathol-012615-044506. Epub 2016 Feb 22. Annu Rev Pathol. 2016. PMID: 26907525 Free PMC article. Review.
References
-
- Boyer SJ (2002) Small molecule inhibitors of KDR (VEGFR-2) kinase: an overview of structure activity relationships. Curr Top Med Chem 2: 973–1000 - PubMed
-
- Brockington A, Lewis C, Wharton S, Shaw PJ (2004) Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol 30: 427–446 - PubMed
-
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583 - PubMed
-
- Choi YB, Kim CK, Yun Y (1999) Lad, an adapter protein interacting with the SH2 domain of p56lck, is required for T cell activation. J Immunol 163: 5242–5249 - PubMed
-
- Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L (2003) Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem 278: 40973–40979 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

