Role of NK-1 neurotransmission in opioid-induced hyperalgesia
- PMID: 15964684
- PMCID: PMC1440305
- DOI: 10.1016/j.pain.2005.04.014
Role of NK-1 neurotransmission in opioid-induced hyperalgesia
Abstract
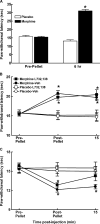
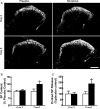
Opiates are among the most important drugs for treatment of moderate to severe pain and prolonged opiate administration is often required to treat chronic pain states. We investigated the neurobiological actions of sustained opiate administration revealing paradoxical pronociceptive adaptations associated with NK-1 receptor function. Sustained morphine delivered over 6 days elicited hyperalgesia in rats and mice during the period of opiate delivery. Sustained morphine administration increased substance P (SP) and NK-1 receptor expression in the spinal dorsal horn. Sustained morphine treatment also enhanced capsaicin-evoked SP release in vitro, and increased internalization of NK-1 receptors in response to noxious stimulation. While NK-1 receptor internalization was observed primarily in the superficial laminae of placebo-treated rats, NK-1 receptor internalization was seen in both superficial and deep lamina of the dorsal horn in morphine-treated animals. Morphine-induced hyperalgesia was reversed by spinal administration of an NK-1 receptor antagonist in rats and mice, and was observed in wildtype (NK-1(+/+)), but not NK-1 receptor knockout (NK-1(-/-)), mice. These data support a critical role for the NK-1 receptor in the expression of sustained morphine-induced hyperalgesia. Additionally, these data indicate that sustained opiate administration induces changes reminiscent of those associated with inflammatory pain. These opiate-induced changes might produce unintended deleterious actions in the course of pain treatment in patients. Understanding of sustained morphine-induced neurochemical changes will help identify approaches that limit the deleterious actions of opiates.
Figures




Similar articles
-
TRPV1 receptor in expression of opioid-induced hyperalgesia.J Pain. 2009 Mar;10(3):243-52. doi: 10.1016/j.jpain.2008.07.004. Epub 2008 Sep 6. J Pain. 2009. PMID: 18774343 Free PMC article.
-
Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways.Pain. 2007 May;129(1-2):35-45. doi: 10.1016/j.pain.2006.09.033. Epub 2006 Nov 22. Pain. 2007. PMID: 17123731 Free PMC article.
-
Loss of neurons in rostral ventromedial medulla that express neurokinin-1 receptors decreases the development of hyperalgesia.Neuroscience. 2013 Oct 10;250:151-65. doi: 10.1016/j.neuroscience.2013.06.057. Epub 2013 Jul 3. Neuroscience. 2013. PMID: 23831426 Free PMC article.
-
Tachykinin NK₁ receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation.Eur J Pharmacol. 2012 Jun 5;684(1-3):64-70. doi: 10.1016/j.ejphar.2012.03.025. Eur J Pharmacol. 2012. PMID: 22724132 Free PMC article.
-
Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance?Neurosignals. 2005;14(4):194-205. doi: 10.1159/000087658. Neurosignals. 2005. PMID: 16215302 Review.
Cited by
-
Effects of opium addiction on level of sensory block in spinal anesthesia with bupivacaine for lower abdomen and limb surgery: a case-control study.Anesth Pain Med. 2014 Nov 26;4(5):e21571. doi: 10.5812/aapm.21571. eCollection 2014 Dec. Anesth Pain Med. 2014. PMID: 25798378 Free PMC article.
-
Acute morphine induces matrix metalloproteinase-9 up-regulation in primary sensory neurons to mask opioid-induced analgesia in mice.Mol Pain. 2012 Mar 25;8:19. doi: 10.1186/1744-8069-8-19. Mol Pain. 2012. PMID: 22444868 Free PMC article.
-
Rescue of HSP70 in Spinal Neurons Alleviates Opioids-Induced Hyperalgesia via the Suppression of Endoplasmic Reticulum Stress in Rodents.Front Cell Dev Biol. 2020 May 12;8:269. doi: 10.3389/fcell.2020.00269. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32500072 Free PMC article.
-
Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway.J Neurosci. 2008 May 28;28(22):5836-45. doi: 10.1523/JNEUROSCI.4170-07.2008. J Neurosci. 2008. PMID: 18509045 Free PMC article.
-
TRPV1 receptor in expression of opioid-induced hyperalgesia.J Pain. 2009 Mar;10(3):243-52. doi: 10.1016/j.jpain.2008.07.004. Epub 2008 Sep 6. J Pain. 2009. PMID: 18774343 Free PMC article.
References
-
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–9. - PubMed
-
- Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–85. - PubMed
-
- Cascieri MA, Macleod AM, Underwood D, Shiao LL, Ber E, Sadowski S, Yu H, Merchant KJ, Swain CJ, Strader CD, Fong TM. Characterization of the interaction of N-acyl-L-tryptophan benzyl ester neurokinin antagonists with the human neurokinin-1 receptor. J Biol Chem. 1994;269:6587–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

