Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein
- PMID: 15964811
- PMCID: PMC1156991
- DOI: 10.1128/MCB.25.13.5552-5566.2005
Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein
Abstract
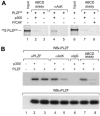
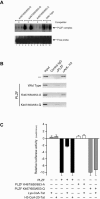
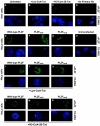
Histone acetyltransferase (HAT) activities of proteins such as p300, CBP, and P/CAF play important roles in activation of gene expression. We now show that the HAT activity of p300 can also be required for down-regulation of transcription by a DNA binding repressor protein. Promyelocytic leukemia zinc finger (PLZF), originally identified as a fusion with retinoic acid receptor alpha in rare cases of all-trans-retinoic acid-resistant acute promyelocytic leukemia, is a transcriptional repressor that recruits histone deacetylase-containing corepressor complexes to specific DNA binding sites. PLZF associates with p300 in vivo, and its ability to repress transcription is specifically dependent on HAT activity of p300 and acetylation of lysines in its C-terminal C2-H2 zinc finger motif. An acetylation site mutant of PLZF does not repress transcription and is functionally deficient in a colony suppression assay despite retaining its abilities to interact with corepressor/histone deacetylase complexes. This is due to the fact that acetylation of PLZF activates its ability to bind specific DNA sequences both in vitro and in vivo. Taken together, our results indicate that a histone deacetylase-dependent transcriptional repressor can be positively regulated through acetylation and point to an unexpected role of a coactivator protein in transcriptional repression.
Figures







Similar articles
-
Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia.Blood. 1998 Apr 15;91(8):2634-42. Blood. 1998. PMID: 9531570
-
Fusion protein of retinoic acid receptor alpha with promyelocytic leukemia protein or promyelocytic leukemia zinc finger protein recruits N-CoR-TBLR1 corepressor complex to repress transcription in vivo.J Biol Chem. 2003 Aug 15;278(33):30788-95. doi: 10.1074/jbc.M303309200. Epub 2003 Jun 5. J Biol Chem. 2003. PMID: 12794076
-
Interaction of EVI1 with cAMP-responsive element-binding protein-binding protein (CBP) and p300/CBP-associated factor (P/CAF) results in reversible acetylation of EVI1 and in co-localization in nuclear speckles.J Biol Chem. 2001 Nov 30;276(48):44936-43. doi: 10.1074/jbc.M106733200. Epub 2001 Sep 21. J Biol Chem. 2001. PMID: 11568182
-
Retinoid receptors in health and disease: co-regulators and the chromatin connection.Semin Cell Dev Biol. 1999 Apr;10(2):215-25. doi: 10.1006/scdb.1999.0303. Semin Cell Dev Biol. 1999. PMID: 10441075 Review.
-
The PLZF gene of t (11;17)-associated APL.Curr Top Microbiol Immunol. 2007;313:31-48. doi: 10.1007/978-3-540-34594-7_3. Curr Top Microbiol Immunol. 2007. PMID: 17217037 Review.
Cited by
-
Analysis of the interaction between Zinc finger protein 179 (Znf179) and promyelocytic leukemia zinc finger (Plzf).J Biomed Sci. 2013 Dec 20;20(1):98. doi: 10.1186/1423-0127-20-98. J Biomed Sci. 2013. PMID: 24359566 Free PMC article.
-
Regulation of the positive transcriptional effect of PLZF through a non-canonical EZH2 activity.Nucleic Acids Res. 2018 Apr 20;46(7):3339-3350. doi: 10.1093/nar/gky080. Nucleic Acids Res. 2018. PMID: 29425303 Free PMC article.
-
Promyelocytic leukemia zinc finger protein regulates interferon-mediated innate immunity.Immunity. 2009 Jun 19;30(6):802-16. doi: 10.1016/j.immuni.2009.04.013. Epub 2009 Jun 11. Immunity. 2009. PMID: 19523849 Free PMC article.
-
Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor.Chem Biol. 2010 May 28;17(5):471-82. doi: 10.1016/j.chembiol.2010.03.006. Chem Biol. 2010. PMID: 20534345 Free PMC article.
-
Modulation of lysine acetylation-stimulated repressive activity by Erk2-mediated phosphorylation of RIP140 in adipocyte differentiation.Cell Signal. 2008 Oct;20(10):1911-9. doi: 10.1016/j.cellsig.2008.07.001. Epub 2008 Jul 5. Cell Signal. 2008. PMID: 18655826 Free PMC article.
References
-
- Aalfs, J. D., and R. E. Kingston. 2000. What does ‘chromatin remodeling’ mean? Trends Biochem. Sci. 25:548-555. - PubMed
-
- Ahmad, K. F., A. Melnick, S. Lax, D. Bouchard, J. Liu, C. L. Kiang, S. Mayer, S. Takahashi, J. D. Licht, and G. G. Prive. 2003. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol. Cell 12:1551-1564. - PubMed
-
- Bannister, A. J., E. A. Miska, D. Gorlich, and T. Kouzarides. 2000. Acetylation of importin-alpha nuclear import factors by CBP/p300. Curr. Biol. 10:467-470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
