Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight
- PMID: 15964817
- PMCID: PMC1157007
- DOI: 10.1128/MCB.25.13.5616-5625.2005
Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight
Abstract
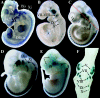
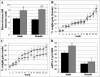
To gain insight into the expression pattern and functional importance of the forkhead transcription factor Foxs1, we constructed a Foxs1-beta-galactosidase reporter gene "knock-in" (Foxs1beta-gal/beta-gal) mouse, in which the wild-type (wt) Foxs1 allele has been inactivated and replaced by a beta-galactosidase reporter gene. Staining for beta-galactosidase activity reveals an expression pattern encompassing neural crest-derived cells, e.g., cranial and dorsal root ganglia as well as several other cell populations in the central nervous system (CNS), most prominently the internal granule layer of cerebellum. Other sites of expression include the lachrymal gland, outer nuclear layer of retina, enteric ganglion neurons, and a subset of thalamic and hypothalamic nuclei. In the CNS, blood vessel-associated smooth muscle cells and pericytes stain positive for Foxs1. Foxs1beta-gal/beta-gal mice perform significantly better (P < 0.01) on a rotating rod than do wt littermates. We have also noted a lower body weight gain (P < 0.05) in Foxs1beta-gal/lbeta-gal males on a high-fat diet, and we speculate that dorsomedial hypothalamic neurons, expressing Foxs1, could play a role in regulating body weight via regulation of sympathetic outflow. In support of this, we observed increased levels of uncoupling protein 1 mRNA in Foxs1beta-gal/beta-gal mice. This points toward a role for Foxs1 in the integration and processing of neuronal signals of importance for energy turnover and motor function.
Figures






Similar articles
-
Emergence of the sensory nervous system as defined by Foxs1 expression.Differentiation. 2007 Jun;75(5):404-17. doi: 10.1111/j.1432-0436.2006.00154.x. Epub 2007 Feb 16. Differentiation. 2007. PMID: 17309606
-
Regulation of the neurofibromatosis 2 gene promoter expression during embryonic development.Dev Dyn. 2006 Oct;235(10):2771-85. doi: 10.1002/dvdy.20883. Dev Dyn. 2006. PMID: 16894610
-
Characterization of lacZ-expressing cells in the gut of embryonic and adult DbetaH-nlacZ mice.J Comp Neurol. 2003 Sep 15;464(2):208-19. doi: 10.1002/cne.10766. J Comp Neurol. 2003. PMID: 12898613
-
The adult hair follicle: cradle for pluripotent neural crest stem cells.Birth Defects Res C Embryo Today. 2004 Jun;72(2):162-72. doi: 10.1002/bdrc.20008. Birth Defects Res C Embryo Today. 2004. PMID: 15269890 Review.
-
Motor columns caged by crest.Trends Neurosci. 2004 Feb;27(2):62-3. doi: 10.1016/j.tins.2003.11.009. Trends Neurosci. 2004. PMID: 15106634 Review.
Cited by
-
Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle.Front Aging Neurosci. 2014 Sep 18;6:245. doi: 10.3389/fnagi.2014.00245. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25278877 Free PMC article. Review.
-
Expression of CD24 in Human Bone Marrow-Derived Mesenchymal Stromal Cells Is Regulated by TGFβ3 and Induces a Myofibroblast-Like Genotype.Stem Cells Int. 2016;2016:1319578. doi: 10.1155/2016/1319578. Epub 2015 Dec 14. Stem Cells Int. 2016. PMID: 26788063 Free PMC article.
-
Pericytes in the eye.Pflugers Arch. 2013 Jun;465(6):789-96. doi: 10.1007/s00424-013-1272-6. Epub 2013 Apr 9. Pflugers Arch. 2013. PMID: 23568370 Review.
-
A comprehensive study of arthropod and onychophoran Fox gene expression patterns.PLoS One. 2022 Jul 8;17(7):e0270790. doi: 10.1371/journal.pone.0270790. eCollection 2022. PLoS One. 2022. PMID: 35802758 Free PMC article.
-
Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner.Stem Cell Res Ther. 2014 Nov 6;5(6):122. doi: 10.1186/scrt512. Stem Cell Res Ther. 2014. PMID: 25376879 Free PMC article.
References
-
- Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421-426. - PubMed
-
- Ang, S. L., and J. Rossant. 1994. HNF-3 beta is essential for node and notochord formation in mouse development. Cell 78:561-574. - PubMed
-
- Bergquist, F., H. N. Shahabi, and H. Nissbrandt. 2003. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 973:81-91. - PubMed
-
- Bernardis, L. L. 1975. The dorsomedial hypothalamic nucleus in autonomic and neuroendocrine homeostasis. Can. J. Neurol. Sci. 2:45-60. - PubMed
-
- Bernardis, L. L., and L. L. Bellinger. 1991. Brown (BAT) and white (WAT) adipose tissue in high-fat junk food (HFJF) and chow-fed rats with dorsomedial hypothalamic lesions (DMNL rats). Behav. Brain Res. 43:191-195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
