Generation of rac3 null mutant mice: role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia
- PMID: 15964830
- PMCID: PMC1157002
- DOI: 10.1128/MCB.25.13.5777-5785.2005
Generation of rac3 null mutant mice: role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia
Abstract
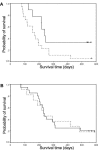
Numerous studies indirectly implicate Rac GTPases in cancer. To investigate if Rac3 contributes to normal or malignant cell function, we generated rac3 null mutants through gene targeting. These mice were viable, fertile, and lacked an obvious external phenotype. This shows Rac3 function is dispensable for embryonic development. Bcr/Abl is a deregulated tyrosine kinase that causes chronic myelogenous leukemia and Ph-positive acute lymphoblastic leukemia in humans. Vav1, a hematopoiesis-specific exchange factor for Rac, was constitutively tyrosine phosphorylated in primary lymphomas from Bcr/Abl P190 transgenic mice, suggesting inappropriate Rac activation. rac3 is expressed in these malignant hematopoietic cells. Using lysates from BCR/ABL transgenic mice that express or lack rac3, we detected the presence of activated Rac3 but not Rac1 or Rac2 in the malignant precursor B-lineage lymphoblasts. In addition, in female P190 BCR/ABL transgenic mice, lack of rac3 was associated with a longer average survival. These data are the first to directly show a stimulatory role for Rac in leukemia in vivo. Moreover, our data suggest that interference with Rac3 activity, for example, by using geranyl-geranyltransferase inhibitors, may provide a positive clinical benefit for patients with Ph-positive acute lymphoblastic leukemia.
Figures





References
-
- Aghazadeh, B., W. E. Lowry, X. Y. Huang, and M. K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102:625-633. - PubMed
-
- Bassermann, F., T. Jahn, C. Miething, P. Seipel, R. Y. Bai, S. Coutinho, V. L. Tybulewicz, C. Peschel, and J. Duyster. 2002. Association of Bcr-Abl with the proto-oncogene Vav is implicated in activation of the Rac-1 pathway. J. Biol. Chem. 277:12437-12445. - PubMed
-
- Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. - PubMed
-
- Bolis, A., S. Corbetta, A. Cioce, and I. de Curtis. 2003. Differential distribution of Rac1 and Rac3 GTPases in the developing mouse brain: implications for a role of Rac3 in Purkinje cell differentiation. Eur. J. Neurosci. 18:2417-2424. - PubMed
-
- Crespo, P., K. E. Schuebel, A. A. Ostrom, J. S. Gutkind, and X. R. Bustelo. 1997. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the Vav proto-oncogene product. Nature 385:169-172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
