Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction
- PMID: 15964918
- PMCID: PMC1618822
- DOI: 10.1152/ajpheart.01088.2004
Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction
Abstract
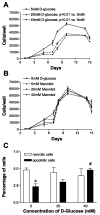
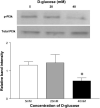
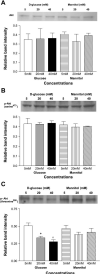
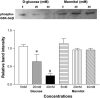
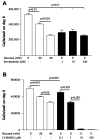
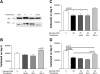
Diabetes mellitus is a major risk factor for the development of vascular complications. We hypothesized that hyperglycemia decreases endothelial cell (EC) proliferation and survival via phosphatidylinositol 3-kinase (PI3k) and Akt signaling pathways. We cultured human umbilical vein ECs (HUVEC) in 5, 20, or 40 mM d-glucose. Cells grown in 5, 20, and 40 mM mannitol served as a control for osmotic effects. We measured EC proliferation for up to 15 days. We assessed apoptosis by annexin V and propidium iodide staining and flow cytometry, analyzed cell lysates obtained on culture day 8 for total and phosphorylated PI3k and Akt by Western blot analysis, and measured Akt kinase activity using a GSK fusion protein. HUVEC proliferation was also tested in the presence of pharmacological inhibitors of PI3k-Akt (wortmannin and LY294002) and after transfection with a constitutively active Akt mutant. ECs in media containing 5 mM d-glucose (control) exhibited log-phase growth on days 7-10. d-Glucose at 20 and 40 mM significantly decreased proliferation versus control (P < 0.05 for both), whereas mannitol did not impair EC proliferation. Apoptosis increased significantly in HUVEC exposed to 40 mM d-glucose. d-Glucose at 40 mM significantly decreased tyrosine-phosphorylated PI3k, threonine 308-phosphorylated-Akt, and Akt activity relative to control 5 mM d-glucose. Pharmacological inhibition of PI3k-Akt resulted in a dose-dependent decrease in EC proliferation. Transfection with a constitutively active Akt mutant protected ECs by enhancing proliferation when grown in 20 and 40 mM d-glucose. We conclude that d-glucose regulates Akt signaling through threonine phosphorylation of Akt and that hyperglycemia-impaired PI3k-Akt signaling may promote EC proliferative dysfunction in diabetes.
Figures
Comment in
-
Does hyperglycemia reduce proliferation or increase apoptosis?Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1486; author reply H1487. doi: 10.1152/ajpheart.00301.2006. Epub 2006 Apr 14. Am J Physiol Heart Circ Physiol. 2006. PMID: 16617134 No abstract available.
Similar articles
-
[High D-glucose alters PI3K and Akt signaling and leads to endothelial cell migration, proliferation and angiogenesis dysfunction].Zhonghua Yi Xue Za Zhi. 2006 Dec 26;86(48):3425-30. Zhonghua Yi Xue Za Zhi. 2006. PMID: 17313857 Chinese.
-
High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway.Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):539-45. doi: 10.1161/01.ATV.0000155462.24263.e4. Epub 2005 Jan 13. Arterioscler Thromb Vasc Biol. 2005. PMID: 15653566
-
Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation.J Biol Chem. 2002 Mar 22;277(12):9684-9. doi: 10.1074/jbc.M106157200. Epub 2002 Jan 4. J Biol Chem. 2002. PMID: 11779850
-
Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner.Circulation. 2007 Oct 2;116(14):1585-95. doi: 10.1161/CIRCULATIONAHA.107.716498. Epub 2007 Sep 17. Circulation. 2007. Retraction in: Circulation. 2020 Oct 13;142(15):e239. doi: 10.1161/CIR.0000000000000919. PMID: 17875968 Retracted.
-
Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications.Physiol Res. 2005;54(1):1-16. doi: 10.33549/physiolres.930582. Physiol Res. 2005. PMID: 15717836 Review.
Cited by
-
Calystegines Improve the Metabolic Activity of Human Adipose Derived Stromal Stem Cells (ASCs) under Hyperglycaemic Condition through the Reduction of Oxidative/ER Stress, Inflammation, and the Promotion of the AKT/PI3K/mTOR Pathway.Biomolecules. 2022 Mar 16;12(3):460. doi: 10.3390/biom12030460. Biomolecules. 2022. PMID: 35327652 Free PMC article.
-
Long-Term Effects of Suramin on Renal Function in Streptozotocin-Induced Diabetes in Rats.Int J Mol Sci. 2023 Sep 28;24(19):14671. doi: 10.3390/ijms241914671. Int J Mol Sci. 2023. PMID: 37834118 Free PMC article.
-
Potential use of transgenic domestic pigs expressing recombinant human erythropoietin in diabetes translation research.Anim Cells Syst (Seoul). 2018 Dec 18;23(1):42-49. doi: 10.1080/19768354.2018.1554544. eCollection 2019 Feb. Anim Cells Syst (Seoul). 2018. PMID: 30834158 Free PMC article.
-
The zebrafish/tumor xenograft angiogenesis assay as a tool for screening anti-angiogenic miRNAs.Cytotechnology. 2015 Dec;67(6):969-75. doi: 10.1007/s10616-014-9735-y. Epub 2014 Jun 20. Cytotechnology. 2015. PMID: 24947063 Free PMC article.
-
Effect of hyperglycemia on human monocyte activation.J Investig Med. 2011 Apr;59(4):661-7. doi: 10.2310/JIM.0b013e31820ee432. J Investig Med. 2011. PMID: 21307779 Free PMC article.
References
-
- Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. - PubMed
-
- Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann NY Acad Sci. 2001;947:93–109. discussion 109–111. - PubMed
-
- Comer GM, Ciulla TA. Pharmacotherapy for diabetic retinopathy. Curr Opin Ophthalmol. 2004;15:508–518. - PubMed
-
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. - PubMed
-
- Curcio F, Ceriello A. Decreased cultured endothelial cell proliferation in high glucose medium is reversed by antioxidants: new insights on the pathophysiological mechanisms of diabetic vascular complications. In Vitro Cell Dev Biol. 1992;28A:787–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

