Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior
- PMID: 15964986
- PMCID: PMC1166597
- DOI: 10.1073/pnas.0501549102
Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior
Abstract
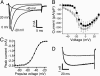
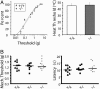
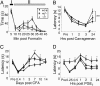
The transmission of pain signals after injury or inflammation depends in part on increased excitability of primary sensory neurons. Nociceptive neurons express multiple subtypes of voltage-gated sodium channels (NaV1s), each of which possesses unique features that may influence primary afferent excitability. Here, we examined the contribution of NaV1.9 to nociceptive signaling by studying the electrophysiological and behavioral phenotypes of mice with a disruption of the SCN11A gene, which encodes NaV1.9. Our results confirm that NaV1.9 underlies the persistent tetrodotoxin-resistant current in small-diameter dorsal root ganglion neurons but suggest that this current contributes little to mechanical thermal responsiveness in the absence of injury or to mechanical hypersensitivity after nerve injury or inflammation. However, the expression of NaV1.9 contributes to the persistent thermal hypersensitivity and spontaneous pain behavior after peripheral inflammation. These results suggest that inflammatory mediators modify the function of NaV1.9 to maintain inflammation-induced hyperalgesia.
Figures
Similar articles
-
Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice.J Physiol. 2006 Oct 1;576(Pt 1):257-67. doi: 10.1113/jphysiol.2006.113597. Epub 2006 Jul 20. J Physiol. 2006. PMID: 16857712 Free PMC article.
-
Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism.J Gen Physiol. 2008 Mar;131(3):211-25. doi: 10.1085/jgp.200709935. Epub 2008 Feb 11. J Gen Physiol. 2008. PMID: 18270172 Free PMC article.
-
Chronic exposure to tumor necrosis factor in vivo induces hyperalgesia, upregulates sodium channel gene expression and alters the cellular electrophysiology of dorsal root ganglion neurons.Neurosci Lett. 2017 Jul 13;653:195-201. doi: 10.1016/j.neulet.2017.05.004. Epub 2017 May 27. Neurosci Lett. 2017. PMID: 28558976
-
NaN/Nav1.9: a sodium channel with unique properties.Trends Neurosci. 2002 May;25(5):253-9. doi: 10.1016/s0166-2236(02)02150-1. Trends Neurosci. 2002. PMID: 11972962 Review.
-
[The role of tetrodotoxin-resistant sodium channels in pain sensation studied on sns-knockout mice].Nihon Rinsho. 2001 Sep;59(9):1688-97. Nihon Rinsho. 2001. PMID: 11554037 Review. Japanese.
Cited by
-
Coping With the Fear of Compartment Syndrome Without Compromising Analgesia: A Narrative Review.Cureus. 2022 Oct 27;14(10):e30776. doi: 10.7759/cureus.30776. eCollection 2022 Oct. Cureus. 2022. PMID: 36447735 Free PMC article. Review.
-
Reduction of voltage gated sodium channel protein in DRG by vector mediated miRNA reduces pain in rats with painful diabetic neuropathy.Mol Pain. 2012 Mar 22;8:17. doi: 10.1186/1744-8069-8-17. Mol Pain. 2012. PMID: 22439790 Free PMC article.
-
Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice.J Physiol. 2006 Oct 1;576(Pt 1):257-67. doi: 10.1113/jphysiol.2006.113597. Epub 2006 Jul 20. J Physiol. 2006. PMID: 16857712 Free PMC article.
-
Heat-resistant action potentials require TTX-resistant sodium channels NaV1.8 and NaV1.9.J Gen Physiol. 2018 Aug 6;150(8):1125-1144. doi: 10.1085/jgp.201711786. Epub 2018 Jul 3. J Gen Physiol. 2018. PMID: 29970412 Free PMC article.
-
Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation.PLoS One. 2011;6(8):e23083. doi: 10.1371/journal.pone.0023083. Epub 2011 Aug 12. PLoS One. 2011. PMID: 21857998 Free PMC article.
References
-
- Kostyuk, P. G., Veselovsky, N. S. & Tsyndrenko, A. Y. (1981) Neuroscience 6, 2423-2430. - PubMed
-
- Kral, M. G., Xiong, Z. & Study, R. E. (1999) Pain 81, 15-24. - PubMed
-
- Zhang, X. F., Zhu, C. Z., Thimmapaya, R., Choi, W. S., Honore, P., Scott, V. E., Kroeger, P. E., Sullivan, J. P., Faltynek, C. R., Gopalakrishnan, M. & Shieh, C. C. (2004) Brain Res. 1009, 147-158. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

