Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways
- PMID: 15964994
- PMCID: PMC1151659
- DOI: 10.1101/gad.1299505
Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways
Abstract
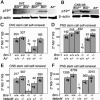
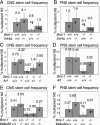
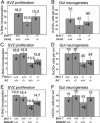
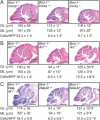
Bmi-1 is required for the post-natal maintenance of stem cells in multiple tissues including the central nervous system (CNS) and peripheral nervous system (PNS). Deletion of Ink4a or Arf from Bmi-1(-/-) mice partially rescued stem cell self-renewal and stem cell frequency in the CNS and PNS, as well as forebrain proliferation and gut neurogenesis. Arf deficiency, but not Ink4a deficiency, partially rescued cerebellum development, demonstrating regional differences in the sensitivity of progenitors to p16Ink4a and p19Arf. Deletion of both Ink4a and Arf did not affect the growth or survival of Bmi-1(-/-) mice or completely rescue neural development. Bmi-1 thus prevents the premature senescence of neural stem cells by repressing Ink4a and Arf, but additional pathways must also function downstream of Bmi-1.
Figures

References
-
- Bixby S., Kruger, G.M., Mosher, J.T., Joseph, N.M., and Morrison, S.J. 2002. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron 35: 643-656. - PubMed
-
- Bruggeman S.W.M., Valk-Lingbeek, M.E., van der Stoop, P.P.M., Jacobs, J.J.L., Kieboom, K., Tanger, E., Hulsman, D., Leung, C., Arsenijevic, Y., Marino, S., and van Lohuizen, M. 2005. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes & Dev. (this issue). - PMC - PubMed
-
- Dimri G.P., Martinez, J.L., Jacobs, J.J., Keblusek, P., Itahana, K., van Lohuizen, M., Campisi, J., Wazer, D.E., and Band, V. 2002. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 62: 4736-4745. - PubMed
-
- Harrison D.E. 1979. Proliferative capacity of erythropoietic stem cell lines and aging: An overview. Mech. Ageing Dev. 9: 409-426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
