Atypical regulation of a green lineage-specific B-type cyclin-dependent kinase
- PMID: 15965018
- PMCID: PMC1176432
- DOI: 10.1104/pp.105.059626
Atypical regulation of a green lineage-specific B-type cyclin-dependent kinase
Abstract
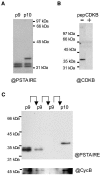
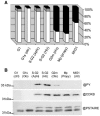
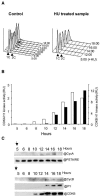
Cyclin-dependent kinases (CDKs) are the main regulators of cell cycle progression in eukaryotes. The role and regulation of canonical CDKs, such as the yeast (Saccharomyces cerevisiae) Cdc2 or plant CDKA, have been extensively characterized. However, the function of the plant-specific CDKB is not as well understood. Besides being involved in cell cycle control, Arabidopsis (Arabidopsis thaliana) CDKB would integrate developmental processes to cell cycle progression. We investigated the role of CDKB in Ostreococcus (Ostreococcus tauri), a unicellular green algae with a minimal set of cell cycle genes. In this primitive alga, at the basis of the green lineage, CDKB has integrated two levels of regulations: It is regulated by Tyr phosphorylation like cdc2/CDKA and at the level of synthesis-like B-type CDKs. Furthermore, Ostreococcus CDKB/cyclin B accounts for the main peak of mitotic activity, and CDKB is able to rescue a yeast cdc28(ts) mutant. By contrast, Ostreococcus CDKA is not regulated by Tyr phosphorylation, and it exhibits a low and steady-state activity from DNA replication to exit of mitosis. This suggests that from a major role in the control of mitosis in green algae, CDKB has evolved in higher plants to assume other functions outside the cell cycle.
Figures




Similar articles
-
Interregulation of CDKA/CDK1 and the Plant-Specific Cyclin-Dependent Kinase CDKB in Control of the Chlamydomonas Cell Cycle.Plant Cell. 2018 Feb;30(2):429-446. doi: 10.1105/tpc.17.00759. Epub 2018 Jan 24. Plant Cell. 2018. PMID: 29367304 Free PMC article.
-
CDKA and CDKB kinases from Chlamydomonas reinhardtii are able to complement cdc28 temperature-sensitive mutants of Saccharomyces cerevisiae.Protoplasma. 2008;232(3-4):183-91. doi: 10.1007/s00709-008-0285-z. Protoplasma. 2008. PMID: 18421551
-
Cell cycle regulated D3-type cyclins form active complexes with plant-specific B-type cyclin-dependent kinase in vitro.Plant Mol Biol. 2006 May;61(1-2):311-27. doi: 10.1007/s11103-006-0014-y. Plant Mol Biol. 2006. PMID: 16786309
-
The plant cell cycle.Annu Rev Plant Biol. 2003;54:235-64. doi: 10.1146/annurev.arplant.54.031902.134836. Annu Rev Plant Biol. 2003. PMID: 14502991 Review.
-
Regulation of cyclin-dependent kinases in Arabidopsis thaliana.Plant Mol Biol. 2000 Aug;43(5-6):583-93. doi: 10.1023/a:1006409907831. Plant Mol Biol. 2000. PMID: 11089862 Review.
Cited by
-
Genomics of Volvocine Algae.Adv Bot Res. 2012;64:185-243. doi: 10.1016/B978-0-12-391499-6.00006-2. Adv Bot Res. 2012. PMID: 25883411 Free PMC article.
-
Phylogenetic analysis of cell-cycle regulatory proteins within the Symbiodiniaceae.Sci Rep. 2020 Nov 24;10(1):20473. doi: 10.1038/s41598-020-76621-1. Sci Rep. 2020. PMID: 33235281 Free PMC article.
-
Use of plankton-derived vitamin B1 precursors, especially thiazole-related precursor, by key marine picoeukaryotic phytoplankton.ISME J. 2017 Mar;11(3):753-765. doi: 10.1038/ismej.2016.145. Epub 2016 Dec 9. ISME J. 2017. PMID: 27935586 Free PMC article.
-
Expression of cell cycle genes in shoot apical meristems.Plant Mol Biol. 2006 Apr;60(6):947-61. doi: 10.1007/s11103-006-0011-1. Plant Mol Biol. 2006. PMID: 16724263 Review.
-
Genome-wide annotation, expression profiling, and protein interaction studies of the core cell-cycle genes in Phalaenopsis aphrodite.Plant Mol Biol. 2014 Jan;84(1-2):203-26. doi: 10.1007/s11103-013-0128-y. Epub 2013 Sep 25. Plant Mol Biol. 2014. PMID: 24222213 Free PMC article.
References
-
- Arellano M, Moreno S (1997) Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol 29: 559–573 - PubMed
-
- Bell MH, Halford NG, Ormrod JC, Francis D (1993) Tobacco plants transformed with cdc25, a mitotic inducer gene from fission yeast. Plant Mol Biol 23: 445–451 - PubMed
-
- Boudolf V, Vlieghe K, Beemster GT, Magyar Z, Acosta JA, Maes S, Van Der Schueren E, Inze D, De Veylder L (2004. b) The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 16: 2683–2692 - PMC - PubMed
-
- Breyne P, Zabeau M (2001) Genome-wide expression analysis of plant cell cycle modulated genes. Curr Opin Plant Biol 4: 136–142 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

