Surrogate splicing for functional analysis of sesquiterpene synthase genes
- PMID: 15965019
- PMCID: PMC1176406
- DOI: 10.1104/pp.105.059386
Surrogate splicing for functional analysis of sesquiterpene synthase genes
Abstract
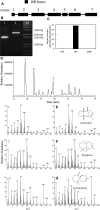
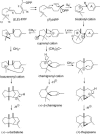
A method for the recovery of full-length cDNAs from predicted terpene synthase genes containing introns is described. The approach utilizes Agrobacterium-mediated transient expression coupled with a reverse transcription-polydeoxyribonucleotide chain reaction assay to facilitate expression cloning of processed transcripts. Subsequent expression of intronless cDNAs in a suitable prokaryotic host provides for direct functional testing of the encoded gene product. The method was optimized by examining the expression of an intron-containing beta-glucuronidase gene agroinfiltrated into petunia (Petunia hybrida) leaves, and its utility was demonstrated by defining the function of two previously uncharacterized terpene synthases. A tobacco (Nicotiana tabacum) terpene synthase-like gene containing six predicted introns was characterized as having 5-epi-aristolochene synthase activity, while an Arabidopsis (Arabidopsis thaliana) gene previously annotated as a terpene synthase was shown to possess a novel sesquiterpene synthase activity for alpha-barbatene, thujopsene, and beta-chamigrene biosynthesis.
Figures



References
-
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267: 730–745 - PubMed
-
- Back K, Chappell J (1995) Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus muticus and its molecular comparison to related terpene cyclases. J Biol Chem 270: 7375–7381 - PubMed
-
- Baynton CE, Potthoff SJ, McCullough AJ, Schuler MA (1996) U-rich tracts enhance 3′ splice site recognition in plant nuclei. Plant J 10: 703–711 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

