Identification of a rat model for usher syndrome type 1B by N-ethyl-N-nitrosourea mutagenesis-driven forward genetics
- PMID: 15965244
- PMCID: PMC1449770
- DOI: 10.1534/genetics.105.044222
Identification of a rat model for usher syndrome type 1B by N-ethyl-N-nitrosourea mutagenesis-driven forward genetics
Abstract
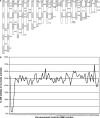
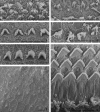
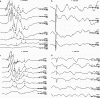
The rat is the most extensively studied model organism and is broadly used in biomedical research. Current rat disease models are selected from existing strains and their number is thereby limited by the degree of naturally occurring variation or spontaneous mutations. We have used ENU mutagenesis to increase genetic variation in laboratory rats and identified a recessive mutant, named tornado, showing aberrant circling behavior, hyperactivity, and stereotypic head shaking. More detailed analysis revealed profound deafness due to disorganization and degeneration of the organ of Corti that already manifests at the onset of hearing. We set up a single nucleotide polymorphism (SNP)-based mapping strategy to identify the affected gene, revealing strong linkage to the central region of chromosome 1. Candidate gene resequencing identified a point mutation that introduces a premature stopcodon in Myo7a. Mutations in human MYO7A result in Usher syndrome type 1B, a severe autosomal inherited recessive disease that involves deafness and vestibular dysfunction. Here, we present the first characterized rat model for this disease. In addition, we demonstrate proof of principle for the generation and cloning of human disease models in rat using ENU mutagenesis, providing good perspectives for systematic phenotypic screens in the rat.
Figures


Similar articles
-
Mutation profile of the MYO7A gene in Spanish patients with Usher syndrome type I.Hum Mutat. 2006 Mar;27(3):290-1. doi: 10.1002/humu.9404. Hum Mutat. 2006. PMID: 16470552
-
Genetic counseling in Usher syndrome: linkage and mutational analysis of 10 Colombian families.Genet Couns. 2008;19(1):15-27. Genet Couns. 2008. PMID: 18564497
-
Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis.Pharmacogenet Genomics. 2006 Mar;16(3):159-69. doi: 10.1097/01.fpc.0000184960.82903.8f. Pharmacogenet Genomics. 2006. PMID: 16495775
-
Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease.Exp Eye Res. 2006 Jul;83(1):97-119. doi: 10.1016/j.exer.2005.11.010. Epub 2006 Mar 20. Exp Eye Res. 2006. PMID: 16545802 Review.
-
Diabetes models by screen for hyperglycemia in phenotype-driven ENU mouse mutagenesis projects.Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E232-40. doi: 10.1152/ajpendo.00592.2007. Epub 2007 Dec 4. Am J Physiol Endocrinol Metab. 2008. PMID: 18056790 Review.
Cited by
-
Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes.J Biomed Sci. 2020 Aug 2;27(1):84. doi: 10.1186/s12929-020-00673-8. J Biomed Sci. 2020. PMID: 32741357 Free PMC article. Review.
-
Myosin7a deficiency results in reduced retinal activity which is improved by gene therapy.PLoS One. 2013 Aug 26;8(8):e72027. doi: 10.1371/journal.pone.0072027. eCollection 2013. PLoS One. 2013. PMID: 23991031 Free PMC article.
-
Myosin VIIa Supports Spermatid/Organelle Transport and Cell Adhesion During Spermatogenesis in the Rat Testis.Endocrinology. 2019 Mar 1;160(3):484-503. doi: 10.1210/en.2018-00855. Endocrinology. 2019. PMID: 30649248 Free PMC article.
-
A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration.Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2097-103. doi: 10.1073/pnas.0812819106. Epub 2009 Feb 5. Proc Natl Acad Sci U S A. 2009. PMID: 19196968 Free PMC article.
-
Insertional mutagenesis by a hybrid piggyBac and sleeping beauty transposon in the rat.Genetics. 2012 Dec;192(4):1235-48. doi: 10.1534/genetics.112.140855. Epub 2012 Sep 28. Genetics. 2012. PMID: 23023007 Free PMC article.
References
-
- Ahmed, Z. M., T. N. Smith, S. Riazuddin, T. Makishima, M. Ghosh et al., 2002. Nonsyndromic recessive deafness DFNB18 and Usher syndrome type IC are allelic mutations of USHIC. Hum. Genet. 110: 527–531. - PubMed
-
- Alagramam, K. N., C. L. Murcia, H. Y. Kwon, K. S. Pawlowski, C. G. Wright et al., 2001. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 27: 99–102. - PubMed
-
- Bockamp, E., M. Maringer, C. Spangenberg, S. Fees, S. Fraser et al., 2002. Of mice and models: improved animal models for biomedical research. Physiol. Genomics 11: 115–132. - PubMed
-
- Brown, S. D., and R. Balling, 2001. Systematic approaches to mouse mutagenesis. Curr. Opin. Genet. Dev. 11: 268–273. - PubMed
-
- Brown, S. D., and P. M. Nolan, 1998. Mouse mutagenesis-systematic studies of mammalian gene function. Hum. Mol. Genet. 7: 1627–1633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

