The effects of flavoxate hydrochloride on voltage-dependent L-type Ca2+ currents in human urinary bladder
- PMID: 15965499
- PMCID: PMC1576239
- DOI: 10.1038/sj.bjp.0706284
The effects of flavoxate hydrochloride on voltage-dependent L-type Ca2+ currents in human urinary bladder
Abstract
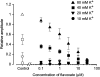
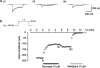
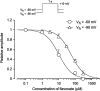
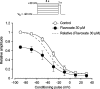
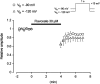
The effects of flavoxate hydrochloride (Bladderon, piperidinoethyl-3-methylflavone-8-carboxylate; hereafter referred as flavoxate) on voltage-dependent nifedipine-sensitive inward Ba(2+) currents in human detrusor myocytes were investigated using a conventional whole-cell patch-clamp. Tension measurement was also performed to study the effects of flavoxate on K(+)-induced contraction in human urinary bladder. Flavoxate caused a concentration-dependent reduction of the K(+)-induced contraction of human urinary bladder. In human detrusor myocytes, flavoxate inhibited the peak amplitude of voltage-dependent nifedipine-sensitive inward Ba(2+) currents in a voltage- and concentration-dependent manner (K(i) = 10 microM), and shifted the steady-state inactivation curve of Ba(2+) currents to the left at a holding potential of -90 mV. Immunohistochemical studies indicated the presence of the alpha(1C) subunit protein, which is a constituent of human L-type Ca(2+) channels (Ca(V)1.2), in the bundles of human detrusor smooth muscle. These results suggest that flavoxate caused muscle relaxation through the inhibition of L-type Ca(2+) channels in human detrusor.
Figures
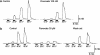


Similar articles
-
Actions of ZD0947, a novel ATP-sensitive K+ channel opener, on membrane currents in human detrusor myocytes.Br J Pharmacol. 2006 Nov;149(5):542-50. doi: 10.1038/sj.bjp.0706893. Epub 2006 Oct 3. Br J Pharmacol. 2006. PMID: 17016513 Free PMC article.
-
Effects of flavoxate hydrochloride on voltage-dependent Ba2+ currents in human detrusor myocytes at different experimental temperatures.Naunyn Schmiedebergs Arch Pharmacol. 2007 Nov;376(3):195-203. doi: 10.1007/s00210-007-0190-6. Epub 2007 Oct 2. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17909749
-
Dual action of AJG049, a novel gut selective Ca2+ channel antagonist, on Ba2+ currents and contractions in guinea-pig antrum myocytes.Eur J Pharmacol. 2009 Mar 1;605(1-3):138-44. doi: 10.1016/j.ejphar.2008.12.039. Epub 2009 Jan 10. Eur J Pharmacol. 2009. PMID: 19168048
-
Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder.Urology. 2006 Aug;68(2):442-8. doi: 10.1016/j.urology.2006.03.039. Urology. 2006. PMID: 16904481
-
Flavoxate.2018 Feb 6. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2018 Feb 6. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31644218 Free Books & Documents. Review.
Cited by
-
Intake of dietary flavonoids in relation to overactive bladder among U.S. adults: a nutritional strategy for improving urinary health.Front Nutr. 2024 Jul 24;11:1437923. doi: 10.3389/fnut.2024.1437923. eCollection 2024. Front Nutr. 2024. PMID: 39114124 Free PMC article.
-
Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F257-F283. doi: 10.1152/ajprenal.00048.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628539 Free PMC article. Review.
-
ATP modulation of osmotically activated anionic current in the membrane of Phycomyces blakesleeanus sporangiophore.Sci Rep. 2023 Jul 24;13(1):11897. doi: 10.1038/s41598-023-39021-9. Sci Rep. 2023. PMID: 37488205 Free PMC article.
-
Actions of ZD0947, a novel ATP-sensitive K+ channel opener, on membrane currents in human detrusor myocytes.Br J Pharmacol. 2006 Nov;149(5):542-50. doi: 10.1038/sj.bjp.0706893. Epub 2006 Oct 3. Br J Pharmacol. 2006. PMID: 17016513 Free PMC article.
-
Natural and synthetic flavonoids, novel blockers of the volume-regulated anion channels, inhibit endothelial cell proliferation.Pflugers Arch. 2018 Oct;470(10):1473-1483. doi: 10.1007/s00424-018-2170-8. Epub 2018 Jun 30. Pflugers Arch. 2018. PMID: 29961148
References
-
- ABBIATI G.A., CESERANI R., NARDI D., PIETRA C., TESTA R. Receptor binding studies of the flavone, REC 15/2053, and other bladder spasmolytics. Pharm. Res. 1988;5:430–433. - PubMed
-
- ANDERSSON K.E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. - PubMed
-
- ANDERSSON K.E. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology. 2004;63:32–41. - PubMed
-
- HAEUSLER G., LEITICH H., VAN TROTSENBURG M., KAIDER A., TEMPFER C.B. Drug therapy of urinary urge incontinence: a systematic review. Obstet. Gynecol. 2002;100:1003–1016. - PubMed
-
- HEGDE S.S., MAMMEN M., JASPER J.R. Antimuscarinics for the treatment of overactive bladder: current options and emerging therapies. Curr. Opin. Invest. Drugs. 2004;5:40–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

