Spatial interference during bimanual coordination: differential brain networks associated with control of movement amplitude and direction
- PMID: 15965999
- PMCID: PMC6871760
- DOI: 10.1002/hbm.20151
Spatial interference during bimanual coordination: differential brain networks associated with control of movement amplitude and direction
Abstract
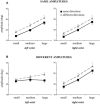
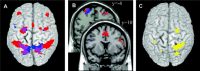
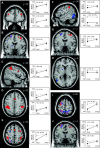
Bimanual interference emerges when spatial features, such as movement direction or amplitude, differ between limbs, as indicated by a mutual bias of limb trajectories. Although first insights into the neural basis of directional interference have been revealed recently, little is known about the neural network associated with amplitude interference. We investigated whether amplitude versus directional interference activates differential networks. Functional magnetic resonance imaging (fMRI) was applied while subjects performed cyclical, bimanual joystick movements with either the same vs. different amplitudes, directions, or both. The kinematic analysis confirmed that subjects experienced amplitude interference when they moved with different as compared to the same amplitude, and directional interference when they moved along different as compared to the same direction. On the brain level, amplitude and directional interference both resulted in activation of a bilateral superior parietal-premotor network, which is known to contribute to sensorimotor transformations during goal-directed movements. Interestingly, amplitude but not directional interference exclusively activated a bilateral network containing the dorsolateral prefrontal cortex, anterior cingulate, and supramarginal gyrus, which was shown previously to contribute to executive functions. Even though the encoding of amplitude and directional information converged and activated the same neural substrate, our data thus show that additional and partly independent mechanisms are involved in bimanual amplitude as compared to that in directional control.
(c) 2005 Wiley-Liss, Inc.
Figures



Similar articles
-
The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour.Eur J Neurosci. 2005 Jul;22(1):235-46. doi: 10.1111/j.1460-9568.2005.04176.x. Eur J Neurosci. 2005. PMID: 16029213
-
Reduced recruitment of motor association areas during bimanual coordination in concert pianists.Hum Brain Mapp. 2004 Jul;22(3):206-15. doi: 10.1002/hbm.20028. Hum Brain Mapp. 2004. PMID: 15195287 Free PMC article.
-
Changes in cerebral activations during movement execution and imagery after parietal cortex TMS interleaved with 3T MRI.Brain Res. 2009 Aug 18;1285:58-68. doi: 10.1016/j.brainres.2009.06.006. Epub 2009 Jun 11. Brain Res. 2009. PMID: 19523932
-
Parietal encoding of action in depth.Neuropsychologia. 2009 May;47(6):1409-20. doi: 10.1016/j.neuropsychologia.2008.12.028. Epub 2008 Dec 30. Neuropsychologia. 2009. PMID: 19154747 Review.
-
Prefrontal and parietal contributions to spatial working memory.Neuroscience. 2006 Apr 28;139(1):173-80. doi: 10.1016/j.neuroscience.2005.04.070. Epub 2005 Dec 2. Neuroscience. 2006. PMID: 16326021 Review.
Cited by
-
Functional MRI evidence for fine motor praxis dysfunction in children with persistent speech disorders.Brain Res. 2015 Feb 9;1597:47-56. doi: 10.1016/j.brainres.2014.11.047. Epub 2014 Dec 4. Brain Res. 2015. PMID: 25481413 Free PMC article.
-
Cerebellar Activation During Simple and Complex Bimanual Coordination: an Activation Likelihood Estimation (ALE) Meta-analysis.Cerebellum. 2022 Dec;21(6):987-1013. doi: 10.1007/s12311-021-01261-8. Epub 2021 Sep 30. Cerebellum. 2022. PMID: 34595608 Review.
-
Alteration of Cortical and Subcortical Structures in Children With Profound Sensorineural Hearing Loss.Front Hum Neurosci. 2020 Dec 9;14:565445. doi: 10.3389/fnhum.2020.565445. eCollection 2020. Front Hum Neurosci. 2020. PMID: 33362488 Free PMC article.
-
Inferior Parietal Lobe Activity Reveals Bimanual Coupling and Interference.Hum Brain Mapp. 2025 Mar;46(4):e70172. doi: 10.1002/hbm.70172. Hum Brain Mapp. 2025. PMID: 40016905 Free PMC article.
-
Haptic feedback helps bipedal coordination.Exp Brain Res. 2016 Oct;234(10):2869-81. doi: 10.1007/s00221-016-4689-2. Epub 2016 Jun 4. Exp Brain Res. 2016. PMID: 27263085 Free PMC article.
References
-
- Alexander GE, Crutcher MD (1990): Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271. - PubMed
-
- Baddeley AD (1986): Working memory. Oxford: Clarendon Press.
-
- Banich MT (1998): The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn 36: 128–157. - PubMed
-
- Bock O, Arnold K (1992): Motor control prior to movement onset: preparatory mechanisms for pointing at visual targets. Exp Brain Res 90: 209–216. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

