The cell cycle-regulated genes of Schizosaccharomyces pombe
- PMID: 15966770
- PMCID: PMC1157095
- DOI: 10.1371/journal.pbio.0030225
The cell cycle-regulated genes of Schizosaccharomyces pombe
Abstract
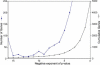
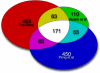
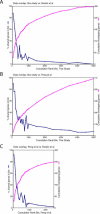
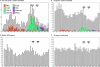
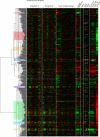
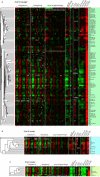
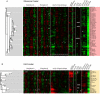
Many genes are regulated as an innate part of the eukaryotic cell cycle, and a complex transcriptional network helps enable the cyclic behavior of dividing cells. This transcriptional network has been studied in Saccharomyces cerevisiae (budding yeast) and elsewhere. To provide more perspective on these regulatory mechanisms, we have used microarrays to measure gene expression through the cell cycle of Schizosaccharomyces pombe (fission yeast). The 750 genes with the most significant oscillations were identified and analyzed. There were two broad waves of cell cycle transcription, one in early/mid G2 phase, and the other near the G2/M transition. The early/mid G2 wave included many genes involved in ribosome biogenesis, possibly explaining the cell cycle oscillation in protein synthesis in S. pombe. The G2/M wave included at least three distinctly regulated clusters of genes: one large cluster including mitosis, mitotic exit, and cell separation functions, one small cluster dedicated to DNA replication, and another small cluster dedicated to cytokinesis and division. S. pombe cell cycle genes have relatively long, complex promoters containing groups of multiple DNA sequence motifs, often of two, three, or more different kinds. Many of the genes, transcription factors, and regulatory mechanisms are conserved between S. pombe and S. cerevisiae. Finally, we found preliminary evidence for a nearly genome-wide oscillation in gene expression: 2,000 or more genes undergo slight oscillations in expression as a function of the cell cycle, although whether this is adaptive, or incidental to other events in the cell, such as chromatin condensation, we do not know.
Figures





References
-
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe . Mol Gen Genet. 1976;146:167–178. - PubMed
-
- Culotti J, Hartwell LH. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp Cell Res. 1971;67:389–401. - PubMed
-
- Hartwell LH. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971;59:183–194. - PubMed
-
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

