Suppression of the pro-apoptotic function of cytochrome c by singlet oxygen via a haem redox state-independent mechanism
- PMID: 15966870
- PMCID: PMC1316276
- DOI: 10.1042/BJ20050580
Suppression of the pro-apoptotic function of cytochrome c by singlet oxygen via a haem redox state-independent mechanism
Abstract
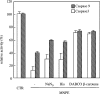
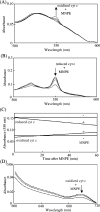
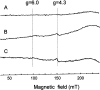
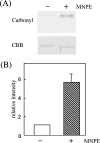
Stimuli for apoptotic signalling typically induce release of cyt c (cytochrome c) from mitochondria. Cyt c then initiates the formation of the apoptosome, comprising Apaf-1 (apoptotic protease-activating factor 1), caspase-9 and other cofactors. The issue of whether the redox state of the haem in cyt c affects the initiation of the apoptotic pathway is currently a subject of debate. In a cell-free reconstitution system, we found that only oxidized cyt c was capable of activating the caspase cascade. Oxidized cyt c was reduced by the physiological reductants cysteine and glutathione, after which it was unable to activate the caspase cascade. It is thus likely that cyt c with oxidized haem is in a conformation capable of interaction with Apaf-1 and forming apoptosomes. When either oxidized or reduced cyt c was treated with submillimolar concentrations of endoperoxide, which affected less than 3% of the redox state of haem, the ability of the oxidized cyt c to activate the caspase cascade was abolished. Higher amounts of singlet oxygen were required to affect the optical spectral change of haem, suggesting that the suppressed pro-apoptotic function of oxidized cyt c is a mechanism that is separate from the redox state of haem. Oxidative protein modification of cyt c by singlet oxygen was evident, on the basis of elevated contents of carbonyl compounds. Our data suggest that singlet oxygen eliminates the pro-apoptotic ability of oxidized cyt c not via the reduction of haem, but via the modification of amino acid residues that are required for apoptosome formation.
Figures





Similar articles
-
An abortive apoptotic pathway induced by singlet oxygen is due to the suppression of caspase activation.Biochem J. 2005 Jul 1;389(Pt 1):197-206. doi: 10.1042/BJ20042067. Biochem J. 2005. PMID: 15796713 Free PMC article.
-
Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine.Free Radic Biol Med. 2004 Dec 15;37(12):1963-85. doi: 10.1016/j.freeradbiomed.2004.08.016. Free Radic Biol Med. 2004. PMID: 15544916 Review.
-
ATP acts as a regulatory effector in modulating structural transitions of cytochrome c: implications for apoptotic activity.Biochemistry. 2009 Apr 21;48(15):3279-87. doi: 10.1021/bi801837e. Biochemistry. 2009. PMID: 19231839
-
Loss of cardiolipin in palmitate-treated GL15 glioblastoma cells favors cytochrome c release from mitochondria leading to apoptosis.J Neurochem. 2008 May;105(3):1019-31. doi: 10.1111/j.1471-4159.2007.05209.x. Epub 2007 Dec 24. J Neurochem. 2008. PMID: 18182042
-
Regulation of apoptosis by the redox state of cytochrome c.Biochim Biophys Acta. 2008 Jul-Aug;1777(7-8):877-81. doi: 10.1016/j.bbabio.2008.03.024. Epub 2008 Apr 3. Biochim Biophys Acta. 2008. PMID: 18439415 Review.
Cited by
-
Evasion of apoptosis as a cellular stress response in cancer.Int J Cell Biol. 2010;2010:370835. doi: 10.1155/2010/370835. Epub 2010 Feb 18. Int J Cell Biol. 2010. PMID: 20182539 Free PMC article.
-
Downregulation of cytochrome c oxidase 1 induced radioresistance in esophageal squamous cell carcinoma.Oncol Lett. 2017 Oct;14(4):4220-4224. doi: 10.3892/ol.2017.6699. Epub 2017 Aug 1. Oncol Lett. 2017. PMID: 28943930 Free PMC article.
-
The effect of iron overload and chelation on erythroid differentiation.Int J Hematol. 2012 Feb;95(2):149-59. doi: 10.1007/s12185-011-0988-3. Epub 2011 Dec 23. Int J Hematol. 2012. PMID: 22193844
-
S. choloroleuca, S. mirzayanii and S. santolinifolia protect PC12 cells from H(2)O (2)-induced apoptosis by blocking the intrinsic pathway.Cytotechnology. 2012 Aug;64(4):403-19. doi: 10.1007/s10616-011-9418-x. Epub 2011 Dec 31. Cytotechnology. 2012. PMID: 22209961 Free PMC article.
-
Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death.Apoptosis. 2010 Apr;15(4):401-11. doi: 10.1007/s10495-009-0436-5. Apoptosis. 2010. PMID: 20091232 Free PMC article.
References
-
- Leist M., Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2001;2:589–598. - PubMed
-
- Green D., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. - PubMed
-
- Hengartner M. O. The biochemistry of apoptosis. Nature (London) 2000;407:770–776. - PubMed
-
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

