Tumor vessel compression hinders perfusion of ultrasonographic contrast agents
- PMID: 15967105
- PMCID: PMC1501164
- DOI: 10.1593/neo.04730
Tumor vessel compression hinders perfusion of ultrasonographic contrast agents
Abstract
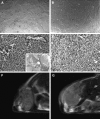
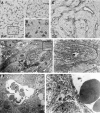
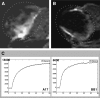
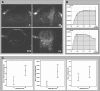
Contrast-enhanced ultrasound (CEUS) is an advanced approach to in vivo assessment of tumor vascularity and is being increasingly adopted in clinical oncology. It is based on 1- to 10 microm-sized gas microbubbles, which can cross the capillary beds of the lungs and are effective echo enhancers. It is known that high cell density, high transendothelial fluid exchange, and poorly functioning lymphatic circulation all provoke solid stress, which compresses vessels and drastically reduces tumor blood flow. Given their size, we supposed that the perfusion of microbubbles is affected by anatomic features of tumor vessels more than are contrast agents traditionally used in dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). Here, we compared dynamic information obtained from CEUS and DCE-MRI on two experimental tumor models exhibiting notable differences in vessel anatomy. We found that tumors with small, flattened vessels show a much higher resistance to microbubble perfusion than to MRI contrast agents, and appear scarcely vascularized at CEUS examination, despite vessel volume adequate for normal function. Thus, whereas CEUS alone could induce incorrect diagnosis when tumors have small or collapsed vessels, integrated analysis using CEUS and DCE-MRI allows in vivo identification of tumors with a vascular profile frequently associated with malignant phenotypes.
Figures

Similar articles
-
Correlation between estimates of tumor perfusion from microbubble contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging.J Ultrasound Med. 2006 Apr;25(4):487-97. doi: 10.7863/jum.2006.25.4.487. J Ultrasound Med. 2006. PMID: 16567438
-
Three-dimensional contrast enhanced ultrasound score and dynamic contrast-enhanced magnetic resonance imaging score in evaluating breast tumor angiogenesis: correlation with biological factors.Eur J Radiol. 2014 Jul;83(7):1098-1105. doi: 10.1016/j.ejrad.2014.03.027. Epub 2014 Apr 12. Eur J Radiol. 2014. PMID: 24794865
-
Contrast-Enhanced Ultrasound with VEGFR2-Targeted Microbubbles for Monitoring Regorafenib Therapy Effects in Experimental Colorectal Adenocarcinomas in Rats with DCE-MRI and Immunohistochemical Validation.PLoS One. 2017 Jan 6;12(1):e0169323. doi: 10.1371/journal.pone.0169323. eCollection 2017. PLoS One. 2017. PMID: 28060884 Free PMC article.
-
Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma.Clin Cancer Res. 2007 Jan 15;13(2 Pt 2):770s-776s. doi: 10.1158/1078-0432.CCR-06-1921. Clin Cancer Res. 2007. PMID: 17255308 Review.
-
Italian Society of Cardiovascular Echography (SIEC) Consensus Conference on the state of the art of contrast echocardiography.Ital Heart J. 2004 Apr;5(4):309-34. Ital Heart J. 2004. PMID: 15185894 Review.
Cited by
-
Epithelial and mesenchymal tumor compartments exhibit in vivo complementary patterns of vascular perfusion and glucose metabolism.Neoplasia. 2007 Nov;9(11):900-8. doi: 10.1593/neo.07541. Neoplasia. 2007. PMID: 18030358 Free PMC article.
-
The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: A prospective study.Eur Radiol. 2018 Apr;28(4):1654-1661. doi: 10.1007/s00330-017-5089-0. Epub 2017 Oct 20. Eur Radiol. 2018. PMID: 29058028
-
HGF/SF increases tumor blood volume: a novel tool for the in vivo functional molecular imaging of Met.Neoplasia. 2006 May;8(5):344-52. doi: 10.1593/neo.05685. Neoplasia. 2006. PMID: 16790083 Free PMC article.
-
In vivo ¹⁸F-FDG tumour uptake measurements in small animals using Cerenkov radiation.Eur J Nucl Med Mol Imaging. 2011 Jan;38(1):120-7. doi: 10.1007/s00259-010-1630-y. Epub 2010 Sep 30. Eur J Nucl Med Mol Imaging. 2011. PMID: 20882278
-
Contrast-enhanced ultrasonography (CEUS) vs. MRI of the small bowel in the evaluation of Crohn's disease activity.Radiol Med. 2012 Mar;117(2):268-81. doi: 10.1007/s11547-011-0783-5. Epub 2012 Jan 21. Radiol Med. 2012. PMID: 22271005
References
-
- Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, Schild HH. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–110. - PubMed
-
- Brasch RC, Li KC, Husband JE, Keogan MT, Neeman M, Padhani AR, Shames D, Turetschek K. In vivo monitoring of tumor angiogenesis with MR imaging. Acad Radiol. 2000;7:812–823. - PubMed
-
- Daldrup-Link HE, Brasch RC. Macromolecular contrast agents for MR mammography: current status. Eur Radiol. 2003;13:354–365. - PubMed
-
- Harvey CJ, Blomley MJ, Eckersley RJ, Cosgrove DO. Development in ultrasound contrast media. Eur Radiol. 2001;11:675–689. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
