Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma
- PMID: 15967106
- PMCID: PMC1501166
- DOI: 10.1593/neo.04685
Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma
Abstract
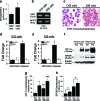
Erythropoietin (Epo) is used for managing anemia in cancer patients. However, recent studies have raised concerns for this practice. We investigated the expression and function of Epo and the erythropoietin receptor (EpoR) in tumor biopsies and cell lines from human head and neck cancer. Epo responsiveness of the cell lines was assessed by Epoetin-alpha-induced tyrosine phosphorylation of the Janus kinase 2 (JAK2) protein kinase. Transmigration assays across Matrigel-coated filters were used to examine the effects of Epoetin-alpha on cell invasiveness. In 32 biopsies, we observed a significant association between disease progression and expression of Epo and its receptor, EpoR. Expression was highest in malignant cells, particularly within hypoxic and infiltrating tumor regions. Although both Epo and EpoR were expressed in human head and neck carcinoma cell lines, only EpoR was upregulated by hypoxia. Epoetin-alpha treatment induced prominent JAK2 phosphorylation and enhanced cell invasion. Inhibition of JAK2 phosphorylation reduced both basal and Epo-induced invasiveness. Our findings support a role for autocrine or paracrine Epo signaling in the malignant progression and local invasiveness of head and neck cancer. This mechanism may also be activated by recombinant Epo therapy and could potentially produce detrimental effects in rhEpo-treated cancer patients.
Figures

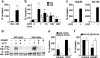
Similar articles
-
Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma.Oncogene. 2005 Jun 23;24(27):4442-9. doi: 10.1038/sj.onc.1208635. Oncogene. 2005. PMID: 15856028
-
Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression.Am J Pathol. 2003 Jun;162(6):1789-806. doi: 10.1016/S0002-9440(10)64314-3. Am J Pathol. 2003. PMID: 12759237 Free PMC article.
-
Survival and invasiveness of astrocytomas promoted by erythropoietin.J Neurosurg. 2007 Feb;106(2):338-50. doi: 10.3171/jns.2007.106.2.338. J Neurosurg. 2007. PMID: 17410721
-
Targeting EPO and EPO receptor pathways in anemia and dysregulated erythropoiesis.Expert Opin Ther Targets. 2016;20(3):287-301. doi: 10.1517/14728222.2016.1090975. Epub 2015 Sep 30. Expert Opin Ther Targets. 2016. PMID: 26419263 Free PMC article. Review.
-
Erythropoietin regulation of red blood cell production: from bench to bedside and back.F1000Res. 2020 Sep 18;9:F1000 Faculty Rev-1153. doi: 10.12688/f1000research.26648.1. eCollection 2020. F1000Res. 2020. PMID: 32983414 Free PMC article. Review.
Cited by
-
Clinical significance of erythropoietin receptor expression in oral squamous cell carcinoma.BMC Cancer. 2012 May 28;12:194. doi: 10.1186/1471-2407-12-194. BMC Cancer. 2012. PMID: 22639817 Free PMC article.
-
A review of the past, present, and future directions of neoplasia.Neoplasia. 2005 Dec;7(12):1039-46. doi: 10.1593/neo.05793. Neoplasia. 2005. PMID: 16354585 Free PMC article. Review. No abstract available.
-
Erythropoietin or Darbepoetin for patients with cancer--meta-analysis based on individual patient data.Cochrane Database Syst Rev. 2009 Jul 8;2009(3):CD007303. doi: 10.1002/14651858.CD007303.pub2. Cochrane Database Syst Rev. 2009. PMID: 19588423 Free PMC article.
-
Longitudinal dynamics of the tumor hypoxia response: From enzyme activity to biological phenotype.Sci Adv. 2023 Nov 24;9(47):eadj6409. doi: 10.1126/sciadv.adj6409. Epub 2023 Nov 22. Sci Adv. 2023. PMID: 37992163 Free PMC article. Review.
-
EPO-R expression patterns in resected gastric adenocarcinoma followed by adjuvant chemoradiation treatment.Pathol Oncol Res. 2009 Mar;15(1):1-10. doi: 10.1007/s12253-008-9118-9. Epub 2008 Nov 11. Pathol Oncol Res. 2009. PMID: 19002606
References
-
- Quirt I, Robeson C, Lau CY, Kovacs M, Burdette-Radoux S, Dolan S, Tang SC, McKenzie M, Couture F Canadian Eprex Oncology Study Group, author. Epoetin alfa therapy increases hemoglobin levels and improves quality of life in patients with cancer-related anemia who are not receiving chemotherapy and patients with anemia who are receiving chemotherapy. J Clin Oncol. 2001;19:4126–4134. - PubMed
-
- Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. - PubMed
-
- Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789–1806. - PMC - PubMed
-
- Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest. 2003;83:1477–1487. - PubMed
-
- Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, et al. Erythropoietin regulates tumor growth, of human malignancies. Carcinogenesis. 2003;24:1021–1029. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
