Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat
- PMID: 15967439
- PMCID: PMC2605388
- DOI: 10.1016/j.expneurol.2005.05.004
Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat
Abstract
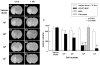
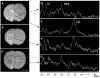
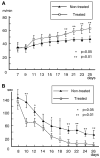
Intravenous infusion of bone marrow cells has demonstrated therapeutic efficacy in animal models of cerebral ischemia and spinal cord injury. We intravenously delivered human mesenchymal stem cells (SH2+, SH3+, CD34-, and CD45-) immortalized with a human-telomerase gene (hTERT-MSCs) and transfected with eGFP or LacZ into rats 12 h after induction of transient middle cerebral artery occlusion (MCAO), to study their potential therapeutic benefit. hTERT-MSCs were delivered at 12 h after lesion induction. Lesion size was assessed using MR imaging and spectroscopy, and histological methods. Functional outcome was assessed using the Morris water maze and a treadmill test. Intravenous delivery of hTERT-MSCs reduced lesion volume and the magnitude of the reduction and functional improvement was positively correlated with the number of cells injected. The reduction of lesion size could be assessed in vivo with MRI and MRS and was correlated with subsequent histological examination of the brain. This work demonstrates that highly purified hTERT-MSCs reduce cerebral infarction volume and improve functional outcome.
Figures

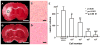

Comment in
-
Bone marrow for the brain?Exp Neurol. 2006 May;199(1):16-9. doi: 10.1016/j.expneurol.2006.03.009. Epub 2006 May 11. Exp Neurol. 2006. PMID: 16696974 No abstract available.
-
Elusive mechanisms of "stem cell"-mediated repair of cerebral damage.Exp Neurol. 2006 May;199(1):10-5. doi: 10.1016/j.expneurol.2006.03.005. Epub 2006 May 30. Exp Neurol. 2006. PMID: 16730352 Review. No abstract available.
References
-
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003 Oct. 30;425:968–973. - PubMed
-
- Barker PB, Gillard JH, van Zijl PC, Soher BJ, Hanley DF, Agildere AM, Oppenheimer SM, Bryan RN. Acute stroke: evaluation with serial proton MR spectroscopic imaging. Radiology. 1994;192(3):723–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

