Fate of biomedical research protocols and publication bias in France: retrospective cohort study
- PMID: 15967761
- PMCID: PMC558532
- DOI: 10.1136/bmj.38488.385995.8F
Fate of biomedical research protocols and publication bias in France: retrospective cohort study
Abstract
Objectives: To describe the fate of protocols approved by the French research ethics committees, a national system created by the French 1988 Huriet-Sérusclat Act; to assess publication bias at a national level.
Design: Retrospective cohort study.
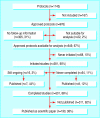
Setting: Representative sample of 25/48 French research ethics committees in 1994. PROTOCOLS: 649 research protocols approved by committees, with follow-up information.
Main outcome measures: Protocols' initial characteristics (design, study size, investigator) abstracted from committees' archives; follow-up information (rates of initiation, completion, and publication) obtained from mailed questionnaire to principal investigators.
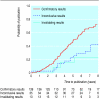
Results: Completed questionnaires were available for 649/976 (69%) protocols. Of these, 581 (90%) studies were initiated, 501/581 (86%) were completed, and 190/501 (38%) were published. Studies with confirmatory results were more likely to be published as scientific papers than were studies with inconclusive results (adjusted odds ratio 4.59, 95% confidence interval 2.21 to 9.54). Moreover, studies with confirmatory results were published more quickly than studies with inconclusive results (hazard ratio 2.48, 1.36 to 4.55).
Conclusion: At a national level, too many research studies are not completed, and among those completed too many are not published. We suggest capitalising on research ethics committees to register and follow all authorised research on human participants on a systematic and prospective basis.
Figures
Comment in
-
Dissemination of results needs to be tracked as well as the funding is.BMJ. 2005 Aug 20;331(7514):456. doi: 10.1136/bmj.331.7514.456-a. BMJ. 2005. PMID: 16110087 Free PMC article. No abstract available.
References
-
- Shields PG. Publication bias is a scientific problem with adverse ethical outcomes: the case for a section for null results. Cancer Epidemiol Biomarkers Prev >2000;9: 771-2. - PubMed
-
- Chalmers I. Underreporting research is scientific misconduct. JAMA >1990;263: 1405-8. - PubMed
-
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA >1990;263: 1385-9. - PubMed
-
- Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol >1986;4: 1529-41. - PubMed
-
- Pich J, Carne X, Arnaiz JA, Gomez B, Trilla A, Rodes J. Role of a research ethics committee in follow-up and publication of results. Lancet >2003;361: 1015-6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources